It’s a scenario familiar to many researchers: you've just completed a meticulous, multi-hour run in your high-temperature furnace. The process was flawless. But as you retrieve the crucible, you either hear the dreaded 'ping' of a stress crack forming, or the data from your final measurement is inexplicably different from the last run. You’re left wondering: was it the sample? The furnace calibration? The balance?
This frustration is more than just an academic puzzle; it’s a significant drain on resources, time, and confidence.
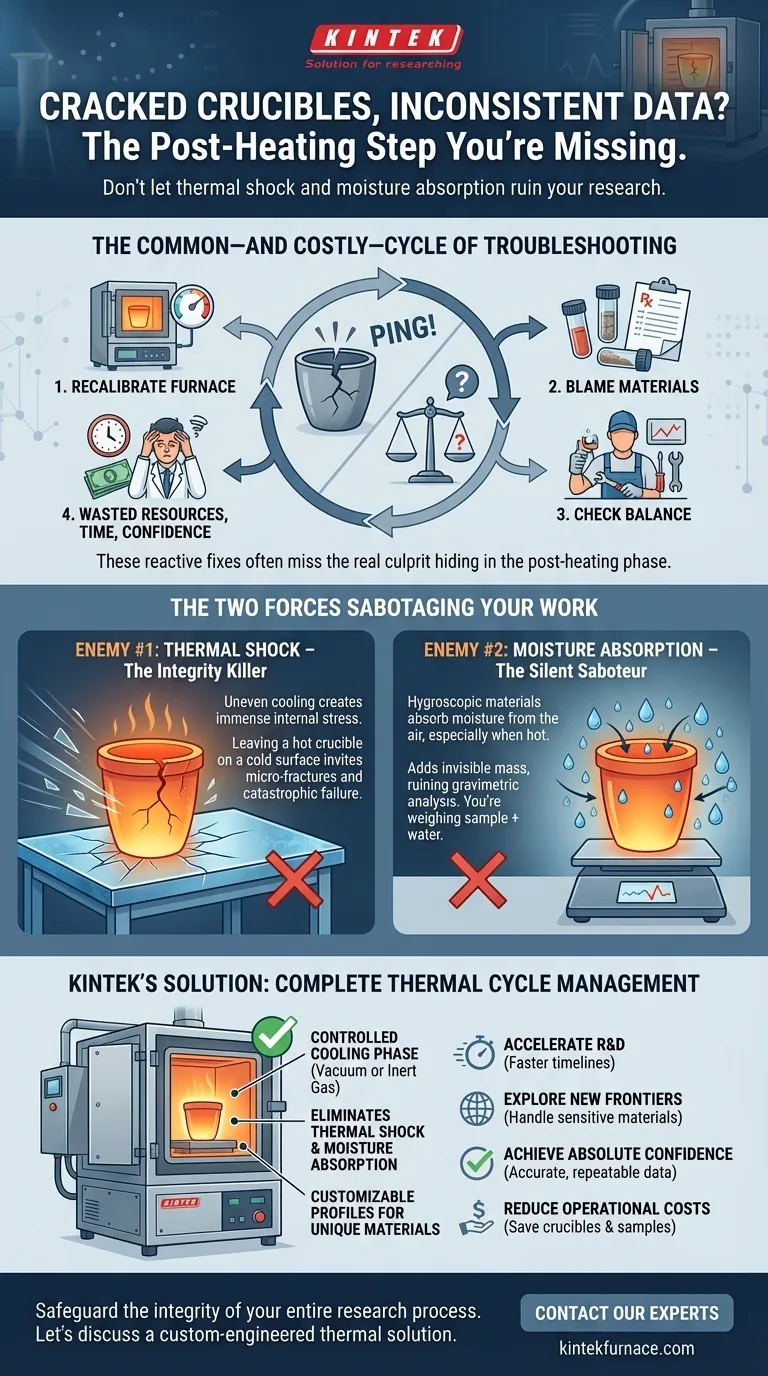
The Common—and Costly—Cycle of Troubleshooting
When faced with cracked crucibles or unreliable data, labs often enter a reactive, expensive troubleshooting loop. The first instinct is usually to blame the most complex piece of equipment.
- "Let's recalibrate the furnace." You spend time and resources ensuring the temperature is perfect, only for the problem to persist.
- "Maybe it's a bad batch of materials." You discard valuable samples and order new ones, delaying the project by days or weeks.
- "The analytical balance must be drifting." You call in a service technician, interrupting lab work, only to be told the equipment is functioning perfectly.
These efforts, while logical, often miss the mark. The problem isn't the heating cycle, the sample purity, or the measurement device. The real culprit is hiding in plain sight, in the deceptively simple minutes after the crucible leaves the furnace. This blind spot leads to costly project delays, squandered R&D budgets, and a critical loss of confidence in your experimental conclusions.
Revealed: The Two Forces Sabotaging Your Work After the Heat is Off
The moment a hot crucible is removed from the controlled interior of a furnace, it's exposed to two powerful, invisible adversaries: thermal shock and atmospheric moisture. Understanding them is the key to solving the problem permanently.
Enemy #1: Thermal Shock – The Integrity Killer
Imagine plunging a hot glass baking dish into cold water. The result is a predictable shatter. The same violent process happens to your crucible, just on a less dramatic scale. When different parts of the crucible cool at different rates, immense internal stresses are created. This uneven cooling, known as thermal shock, is the primary cause of the micro-fractures that eventually lead to catastrophic failure. Leaving a crucible on a cold metal benchtop is a direct invitation for thermal shock to destroy your equipment.
Enemy #2: Moisture Absorption – The Silent Saboteur
Many ceramic materials are hygroscopic, meaning they readily absorb water from the air. This effect is dramatically amplified when the material is hot. To the air in your lab, a hot crucible looks like a thirsty sponge. As it cools in open air, it absorbs moisture, invisibly adding mass. For any process that relies on precise gravimetric analysis, this is a disaster. You aren't weighing your sample; you're weighing your sample plus an unknown amount of water, rendering your data inaccurate and non-repeatable.
This is why common "solutions" fail. They treat symptoms, not the disease. You can't fix a weight-gain problem by recalibrating a scale if the object itself is changing weight. You can't prevent cracking by adjusting furnace temperature if the damage happens after the heating is done.
Beyond Just Heating: A System for the Entire Thermal Cycle
To truly defeat thermal shock and moisture absorption, you need to control the environment not just during heating, but during the critical cooling phase as well. This requires shifting your thinking from buying a "heater" to implementing a complete "thermal processing system."
A truly effective solution must provide a controlled, predictable, and inert environment where a crucible can cool down slowly and evenly, completely shielded from ambient air.
This is precisely the principle behind KINTEK's advanced furnace designs. Our Vacuum & Atmosphere Furnaces are not just engineered for exceptional heating performance; they are designed to manage the full thermal cycle. By allowing the crucible to cool under vacuum or in a controlled inert gas atmosphere, our systems completely eliminate the risks of both moisture absorption and drastic thermal shock.
Furthermore, because different materials have vastly different thermal properties, our deep customization capability is critical. We don't offer a one-size-fits-all box. We work with you to engineer a system—from the heating elements to the programmable cooling profiles—that is precisely tailored to the demands of your unique materials, ensuring their integrity from start to finish.
From Fighting Problems to Fueling Innovation
When you no longer have to worry about crucibles cracking or data being compromised by environmental variables, your lab's potential is transformed. The resources once spent on re-running failed experiments can now be invested in groundbreaking research.
- Accelerate R&D: Drastically shorten project timelines by eliminating the need for repetitive validation runs.
- Explore New Frontiers: Confidently work with highly sensitive or brittle advanced materials that were previously too challenging to handle.
- Achieve Absolute Confidence: Produce data that is not just acceptable, but verifiably accurate, repeatable, and reliable, forming a solid foundation for new products and discoveries.
- Reduce Operational Costs: Save significant budget by minimizing the consumption of expensive crucibles and valuable sample materials.
Solving the crucible cooling challenge isn't just about protecting a piece of ceramic; it's about safeguarding the integrity of your entire research and development process. True experimental success comes from a thermal system that is optimized for every step. If you're ready to move beyond fighting unpredictable results and start building a more robust and efficient process, our team is here to help. Let's discuss how a custom-engineered thermal solution can overcome your specific challenges. Contact Our Experts.
Visual Guide
Related Products
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- Multi Zone Laboratory Quartz Tube Furnace Tubular Furnace
Related Articles
- Why Your High-Temperature Furnace Fails: It’s Not the Heating Element, It’s the Physics
- Beyond the Program: Why Your Sintering Fails and How to Guarantee Uniformity
- Gravity and Heat: The Elegant Engineering of the Drop Tube Furnace
- Why Your High-Temperature Synthesis Results Are Unreliable—And How to Fix It
- Why Your High-Temperature Experiments Fail—And How to Fix Them for Good




















