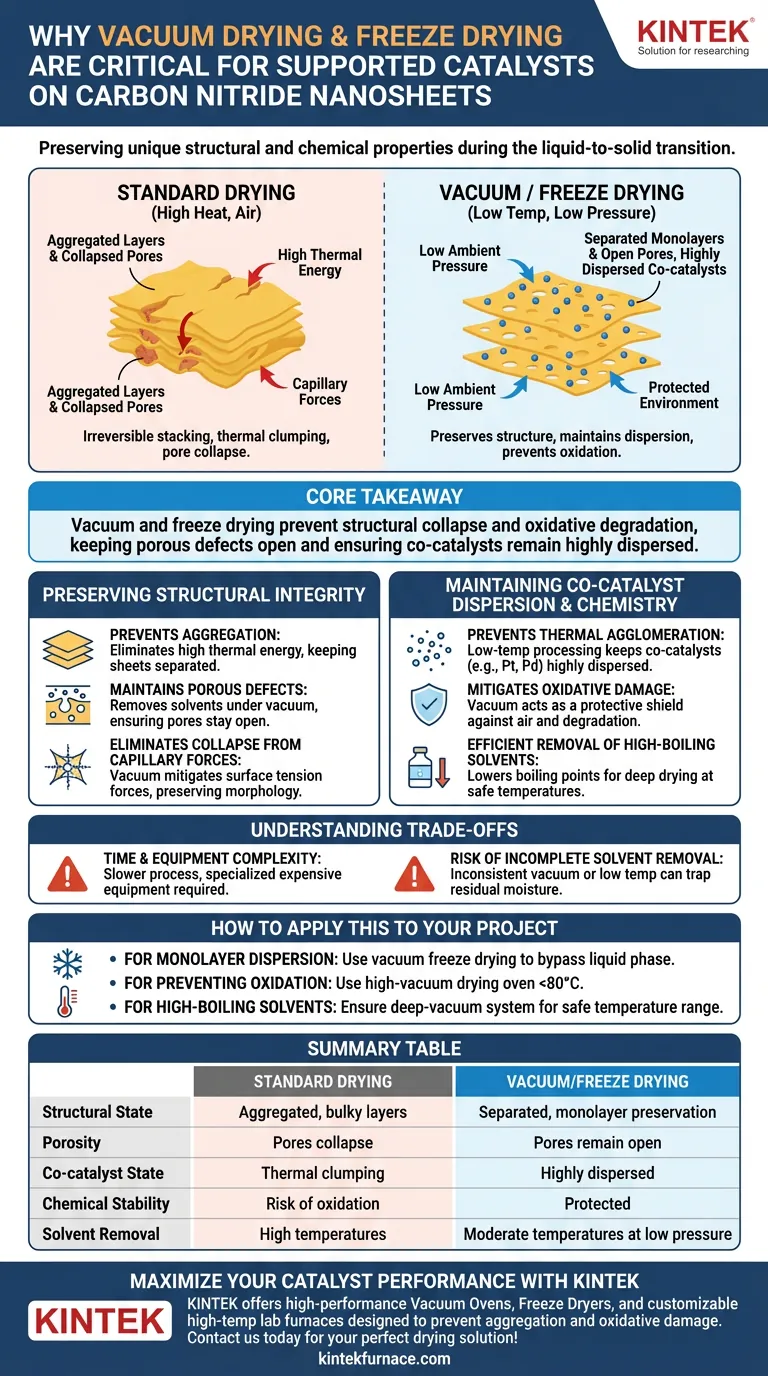
Vacuum drying technology is indispensable for carbon nitride nanosheets because it preserves their unique structural and chemical properties during the critical transition from liquid to solid. By lowering the ambient pressure, these systems allow for the removal of water or organic solvents at significantly reduced temperatures. This process prevents the irreversible stacking of nanosheets and the thermal clumping of co-catalysts, ensuring the final material maintains its high catalytic activity.
Core Takeaway: Vacuum drying and freeze drying are necessary to prevent the structural collapse and oxidative degradation of nanosheets. By removing solvents at low temperatures, these methods keep porous defects open and ensure co-catalysts remain highly dispersed on the material's surface.

Preserving the Structural Integrity of Nanosheets
Prevention of Nanosheet Aggregation
Standard drying processes often cause monolayer carbon nitride nanosheets to stack together into bulkier, less active structures. Vacuum drying eliminates the high thermal energy that typically drives this severe aggregation, allowing the sheets to remain separated.
Maintaining Porous Defects and Surface Area
The "in-plane" porous defects within carbon nitride are vital for transport and reaction kinetics. Removing solvents under vacuum ensures these pores stay open and accessible, rather than being crushed or filled during a high-heat evaporation process.
Eliminating Structural Collapse from Capillary Forces
As liquid evaporates in a standard environment, surface tension creates strong capillary forces that can cause nanomaterials to collapse. Vacuum environments, particularly in freeze drying, mitigate these forces, preserving the loose, porous morphology of the nanosheets.
Maintaining Co-catalyst Dispersion and Chemistry
Preventing Thermal Agglomeration of Nanoparticles
Supported co-catalysts like Platinum (Pt) or Palladium (Pd) are highly sensitive to heat, which causes them to migrate and form large, inactive clumps. Low-temperature vacuum processing ensures these active components remain highly dispersed as individual nanoparticles across the nanosheet surface.
Mitigating Oxidative Damage and Decomposition
Many high-activity catalysts are prone to oxidation or phase transformation when heated in the presence of air. The vacuum environment acts as a protective shield, preventing unintended chemical reactions or the degradation of functional groups during the drying stage.
Efficient Removal of High-Boiling Point Solvents
Solvents like ethylene glycol or anhydrous methanol can be difficult to remove without excessive heat. Reducing the ambient pressure lowers the boiling point of these liquids, allowing for deep drying and the removal of residual acids or organics at safe, moderate temperatures.
Understanding the Trade-offs
Time and Equipment Complexity
Vacuum drying and freeze drying are significantly slower than standard oven drying and require specialized, more expensive equipment. The precision required to maintain vacuum levels and controlled temperatures adds a layer of operational complexity to the catalyst preparation workflow.
Risk of Incomplete Solvent Removal
If the vacuum level is inconsistent or the temperature is set too low for a specific solvent's vapor pressure, residual moisture may remain trapped in the deep pores. This residual solvent can interfere with subsequent grinding or lead to "hard agglomeration" if the material is later exposed to higher temperatures.
How to Apply This to Your Project
Selecting the Right Drying Strategy
- If your primary focus is preserving monolayer dispersion: Use vacuum freeze drying to completely bypass the liquid phase and eliminate capillary-induced stacking.
- If your primary focus is preventing co-catalyst oxidation: Utilize a high-vacuum drying oven at temperatures below 80°C to remove solvents while minimizing oxygen exposure.
- If your primary focus is removing high-boiling point organic solvents: Ensure your vacuum system is rated for deep-vacuum levels to lower the solvent's boiling point to a safe thermal range for the precursor.
By precisely controlling the environment during the drying phase, you ensure that the sophisticated architecture of your carbon nitride catalyst survives the transition from synthesis to application.
Summary Table:
| Feature | Standard Drying | Vacuum/Freeze Drying |
|---|---|---|
| Structural State | Aggregated, bulky layers | Separated, monolayer preservation |
| Porosity | Pores collapse due to capillary force | Pores remain open and accessible |
| Co-catalyst State | Thermal clumping/agglomeration | Highly dispersed nanoparticles |
| Chemical Stability | Risk of oxidation/degradation | Protected by oxygen-free environment |
| Solvent Removal | Requires high temperatures | Moderate temperatures at low pressure |
Maximize Your Catalyst Performance with KINTEK
Precision matters when your research depends on the structural integrity of carbon nitride nanosheets. Backed by expert R&D and manufacturing, KINTEK offers high-performance Vacuum Ovens, Freeze Dryers, and customizable high-temp lab furnaces designed to prevent aggregation and oxidative damage during critical drying stages.
Whether you need CVD systems for synthesis or vacuum drying for dispersion, our equipment is tailored to your unique lab requirements. Contact us today to find the perfect drying solution for your project!
Visual Guide
References
- New Insights In‐Plane Porous Defects Formation Mechanism of Single‐Layer Graphitic Carbon Nitride by Tetrahydrofuran Etching Reaction. DOI: 10.1002/sstr.202500259
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Vacuum Heat Treat Sintering and Brazing Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Dental Porcelain Sintering Furnace for Dental Laboratories
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
People Also Ask
- What are the typical steps in vacuum sintering? Master High-Purity, Dense Material Production
- How does a chiller protect the vacuum furnace itself? Extend Equipment Life with Effective Cooling
- Why has vacuum heat treatment technology gained widespread use? Achieve Superior Material Control and Performance
- What role do high-temperature vacuum furnaces play in CVD and PVD processes? Essential for Purity and Precision in Thin-Film Deposition
- What types of metallurgical processes can vacuum furnaces perform? Achieve Purity and Precision in Metal Treatment
- What advantages does vacuum hardening offer? Achieve Superior, Distortion-Free Heat Treatment
- What advantages does a laboratory vacuum oven offer over a conventional oven for Pb SA/OSC catalyst drying?
- What role do high-temperature furnaces play in Ti-15Mo heat treatment? Unlock Advanced Alloy Performance



















