Choosing the right crucible is paramount for any induction furnace operation because it serves as the primary interface between the furnace's energy and the metal being melted. An incorrect choice can lead to catastrophic failure, melt contamination, and significant financial loss. The crucible must not only contain the liquid metal at extreme temperatures but also resist intense thermal shock and remain chemically non-reactive with the specific alloy being processed.
The crucible is not merely a passive container; it is an active component in the metallurgical process. Its material properties directly dictate the purity of the final product, the safety of the operation, and the overall efficiency of the furnace.

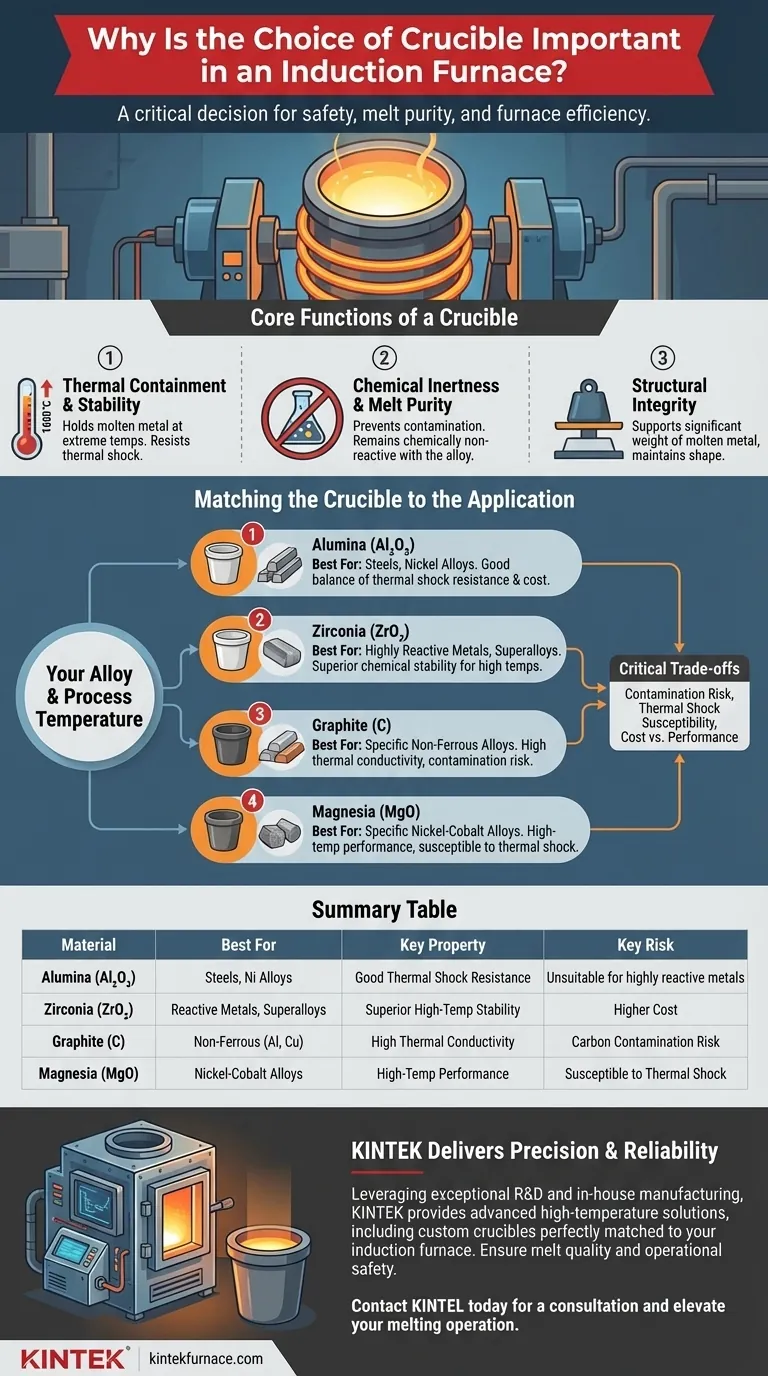
The Core Functions of a Crucible in Induction Melting
To understand the importance of crucible selection, you must first appreciate the demanding roles it plays. The choice is a balance of thermal, chemical, and structural properties tailored to a specific application.
Thermal Containment and Stability
A crucible's most basic function is to hold molten metal at temperatures that can exceed 1600°C (2900°F). It must do this without melting, deforming, or cracking. This requires a material with an exceptionally high melting point and low thermal expansion.
Furthermore, the rapid heating and cooling cycles inherent to induction melting create immense thermal stress. The ability to withstand this rapid temperature change without fracturing is known as thermal shock resistance, a critical property for any crucible.
Chemical Inertness and Melt Purity
Molten metals are highly reactive. A crucible made from the wrong material will chemically react with the melt, leaching impurities into the alloy and altering its final properties. This is a critical failure.
For example, using a graphite crucible to melt certain types of steel can introduce carbon into the alloy, forming unwanted carbides and rendering the entire batch useless. The goal is chemical inertness—the crucible must be a neutral vessel that does not contaminate the product.
Structural Integrity
The crucible must support the significant weight of the molten metal charge. It must maintain its structural integrity even when superheated, ensuring the melt remains safely contained throughout the pouring and casting process.
Matching the Crucible to the Application
The correct crucible material is dictated entirely by the metal being melted and the target process temperature. There is no single "best" material.
For Steels and Nickel-Based Alloys
Alumina (Aluminum Oxide) is a common and effective choice for melting most steels and many nickel-based alloys. It offers a good balance of high-temperature stability, thermal shock resistance, and chemical inertness for these applications.
For High-Temperature and Reactive Metals
When working with highly reactive metals like titanium or superalloys at extreme temperatures, zirconia (Zirconium Dioxide) is often required. It provides superior chemical stability and a higher melting point than alumina, preventing contamination of these sensitive and expensive alloys.
For Specific Non-Ferrous and Other Applications
Graphite crucibles are used for some non-ferrous metals like aluminum and copper alloys, but careful consideration is required to prevent contamination. Magnesia is used for specific nickel-cobalt alloy applications but can be more susceptible to thermal shock if not handled properly.
Understanding the Trade-offs
Selecting a crucible involves navigating a series of critical trade-offs. Misunderstanding these can lead to process failure.
The Risk of Contamination
The most severe consequence of a poor choice is melt contamination. As mentioned, a graphite crucible can ruin a low-carbon steel melt. Similarly, an alumina crucible may not be suitable for highly reactive metals that can strip oxygen from the oxide ceramic, leading to impurities.
Susceptibility to Thermal Shock
Even a chemically compatible crucible can fail. For instance, a magnesia crucible might be the perfect choice chemically but will crack if heated or cooled too quickly. The operator's procedure is just as important as the material itself.
Cost vs. Performance
There is a significant cost difference between materials. A standard clay-graphite or alumina crucible is far less expensive than a high-purity zirconia crucible. The choice becomes an economic calculation: the cost of the crucible must be weighed against the value of the alloy and the risk of a failed melt.
Making the Right Choice for Your Melt
Your selection must be a deliberate decision based on your specific metallurgical goal. Use these principles as your guide.
- If your primary focus is melting standard steels or nickel alloys: Alumina crucibles provide the most reliable and cost-effective balance of performance and chemical stability.
- If your primary focus is melting highly reactive or very high-temperature metals: Invest in a zirconia crucible to ensure the chemical purity and integrity of your final product.
- If your primary focus is melting specific non-ferrous alloys: You may consider graphite or other specialized materials, but you must first verify their chemical compatibility to avoid contaminating your melt.
Ultimately, a properly selected crucible is the foundation for a safe, efficient, and successful melting operation.
Summary Table:
| Crucible Material | Best For | Key Property | Key Risk |
|---|---|---|---|
| Alumina (Al₂O₃) | Steels, Nickel Alloys | Good thermal shock resistance, chemical inertness | Unsuitable for highly reactive metals |
| Zirconia (ZrO₂) | Reactive Metals (e.g., Ti), Superalloys | Superior high-temperature stability | Higher cost |
| Graphite (C) | Specific Non-Ferrous (e.g., Al, Cu) | High thermal conductivity | Carbon contamination risk |
| Magnesia (MgO) | Specific Nickel-Cobalt Alloys | High-temperature performance | Susceptible to thermal shock |
Maximize Your Melting Process with the Right Crucible
Choosing the correct crucible is not a one-size-fits-all decision; it's a precise calculation based on your specific alloy, temperature, and purity requirements. A wrong choice risks costly contamination and equipment failure.
KINTEK delivers precision and reliability. Leveraging our exceptional R&D and in-house manufacturing capabilities, we provide advanced high-temperature furnace solutions, including crucibles perfectly matched to your induction furnace. Our deep customization expertise ensures your crucible delivers the exact thermal stability, chemical inertness, and structural integrity your process demands.
Don't leave your melt quality to chance. Let our experts help you select the ideal crucible to protect your valuable materials and ensure operational safety.
Contact KINTEL today for a consultation and elevate your melting operation.
Visual Guide
Related Products
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Chairside Dental Porcelain Zirconia Sintering Furnace with Transformer for Ceramic Restorations
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- Dental Porcelain Zirconia Sintering Ceramic Vacuum Press Furnace
- Vacuum Induction Melting Furnace
People Also Ask
- How do roller kilns and tube furnaces differ in their use of Alumina ceramic tubes? Compare Transport vs. Containment
- What are the material requirements for furnace tubes? Optimize Performance and Safety in High-Temperature Labs
- How does a tube heating furnace facilitate the carbon coating process? Boost Layered Oxide Conductivity
- What is flash vacuum pyrolysis and how is a tube furnace utilized in this process? Unlock High-Temp Chemical Reactions
- What function does a tube furnace serve in the PVT growth of J-aggregate molecular crystals? Mastery of Thermal Control



















