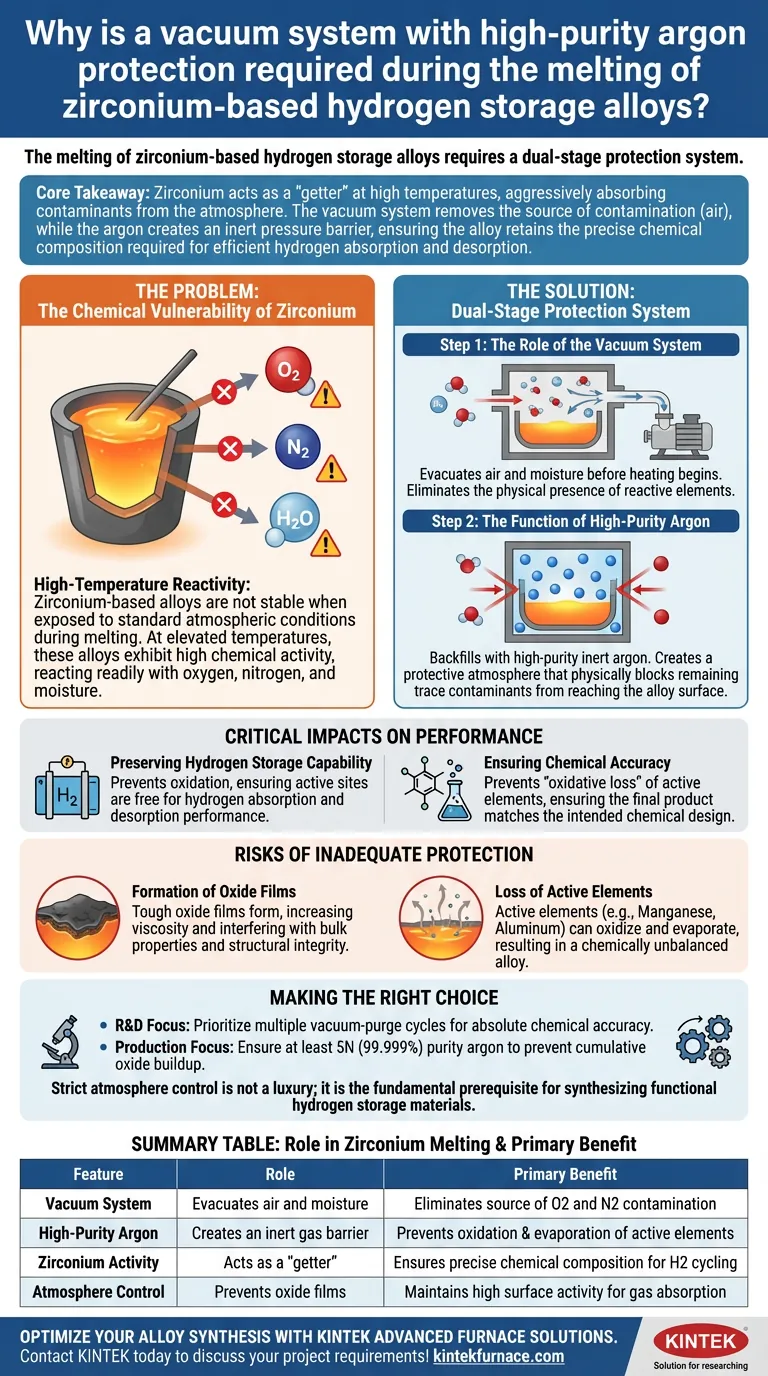
The melting of zirconium-based hydrogen storage alloys requires a dual-stage protection system because Zirconium is exceptionally chemically active at high temperatures. Without a vacuum to evacuate air and high-purity argon to act as a shield, the molten alloy would rapidly react with oxygen, nitrogen, and water vapor, destroying the material's ability to function.
Core Takeaway Zirconium acts as a "getter" at high temperatures, aggressively absorbing contaminants from the atmosphere. The vacuum system removes the source of contamination (air), while the argon creates an inert pressure barrier, ensuring the alloy retains the precise chemical composition required for efficient hydrogen absorption and desorption.
The Chemical Vulnerability of Zirconium
High-Temperature Reactivity
Zirconium-based alloys are not stable when exposed to standard atmospheric conditions during melting.
At elevated temperatures, these alloys exhibit high chemical activity, reacting readily with oxygen, nitrogen, and moisture.
The Role of the Vacuum System
The first line of defense is the vacuum system.
Before heating begins, the furnace chamber must be evacuated to remove air and residual moisture.
This step eliminates the physical presence of reactive elements that would otherwise bond with the Zirconium.
The Function of High-Purity Argon
Once the air is removed, the chamber is backfilled with high-purity argon.
Argon is an inert gas, meaning it does not react chemically with the molten metal.
This creates a protective atmosphere that physically blocks any remaining trace contaminants from reaching the alloy surface.
Critical Impacts on Performance
Preserving Hydrogen Storage Capability
The primary purpose of these alloys is to absorb and desorb hydrogen.
If the alloy oxidizes during melting, the active sites on the material's surface become blocked by oxide layers.
According to the primary technical data, preventing this contamination is essential to maintaining the alloy's subsequent hydrogen absorption and desorption performance.
Ensuring Chemical Accuracy
Alloy performance relies on a precise ratio of elements.
Without an inert atmosphere, active elements within the mix can suffer from "oxidative loss," essentially burning off during the melt.
Argon protection ensures the final product matches the intended chemical design, preventing shifts in phase transformation temperatures.
Understanding the Risks of Inadequate Protection
Formation of Oxide Films
If oxygen is not completely excluded, tough oxide films can form on the surface of the melt.
As noted in comparative metal studies, these films have high viscosity and interfere with the bulk properties of the metal.
This can lead to inconsistent measurement data and structural weaknesses in the solidified alloy.
Loss of Active Elements
Zirconium is often alloyed with other active elements (like Manganese or Aluminum) to tune performance.
These elements are also prone to oxidation and evaporation at high heat.
Failing to use high-purity argon results in an alloy that is chemically unbalanced, potentially rendering it useless for hydrogen storage applications.
Making the Right Choice for Your Goal
To ensure the integrity of your zirconium-based alloys, consider the following operational priorities:
- If your primary focus is Research & Development: Prioritize multiple vacuum-purge cycles before melting to guarantee the absolute chemical accuracy of your multi-component design.
- If your primary focus is Production Efficiency: Ensure your argon supply is at least 5N (99.999%) purity to prevent cumulative oxide buildup that degrades hydrogen cycling performance over time.
Strict atmosphere control is not a luxury; it is the fundamental prerequisite for synthesizing functional hydrogen storage materials.
Summary Table:
| Feature | Role in Zirconium Melting | Primary Benefit |
|---|---|---|
| Vacuum System | Evacuates air and moisture from the furnace | Eliminates source of O2 and N2 contamination |
| High-Purity Argon | Creates an inert gas pressure barrier | Prevents oxidation and evaporation of active elements |
| Zirconium Activity | Acts as a "getter" at high temperatures | Ensures precise chemical composition for H2 cycling |
| Atmosphere Control | Prevents formation of viscous oxide films | Maintains high surface activity for gas absorption |
Optimize Your Alloy Synthesis with KINTEK Advanced Furnace Solutions
Don't let atmospheric contamination compromise your research or production quality. KINTEK provides industry-leading Vacuum, CVD, and customizable high-temperature furnace systems specifically designed to handle chemically active materials like zirconium.
Why partner with KINTEK?
- Expert R&D & Manufacturing: Precision-engineered systems for the most demanding lab environments.
- Total Atmosphere Control: Superior vacuum integrity and high-purity gas delivery systems.
- Customizable Solutions: Tailored configurations for Muffle, Tube, Rotary, and Vacuum systems to meet your unique metallurgical needs.
Ensure your alloys retain maximum hydrogen absorption performance. Contact KINTEK today to discuss your project requirements!
Visual Guide
References
- Achieving Anti‐Disproportionation Performance Enhancement and Distorted Inverse‐Disproportionation Reaction Correction of Zr<sub>2</sub>Fe‐Based Hydrogen Isotope Storage Alloys via Element Substitution. DOI: 10.1002/advs.202507722
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Induction Melting Furnace
- Dental Porcelain Zirconia Sintering Ceramic Vacuum Press Furnace
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Chairside Dental Porcelain Zirconia Sintering Furnace with Transformer for Ceramic Restorations
People Also Ask
- Why is the Vacuum Degassing process critical when refining liquid H13 tool steel? Ensure Purity and Durability
- Why is a vacuum drying oven critical for EN-LCNF carbon electrodes? Achieve Precise Solvent Removal and Zero Oxidation
- Why must sintering furnaces for high-entropy diboride ceramics have vacuum control? Protect Your Material Integrity
- How does a high-temperature vacuum furnace facilitate the synthesis of graphene? Master Precise Catalyst Mediation
- What is the contamination risk difference between low vacuum and high vacuum furnaces? Choose the Right Furnace for Your Lab
- How does the negative pressure environment of a vacuum infiltration furnace improve composites? Achieve 100% Density
- What is the core role of a vacuum resistance melting furnace in the vacuum refining process of AM60 magnesium alloy? Mastering Magnesium's Volatile Nature
- What are the key components of a vacuum system for heat treatment? Achieve Superior Metallurgical Outcomes



















