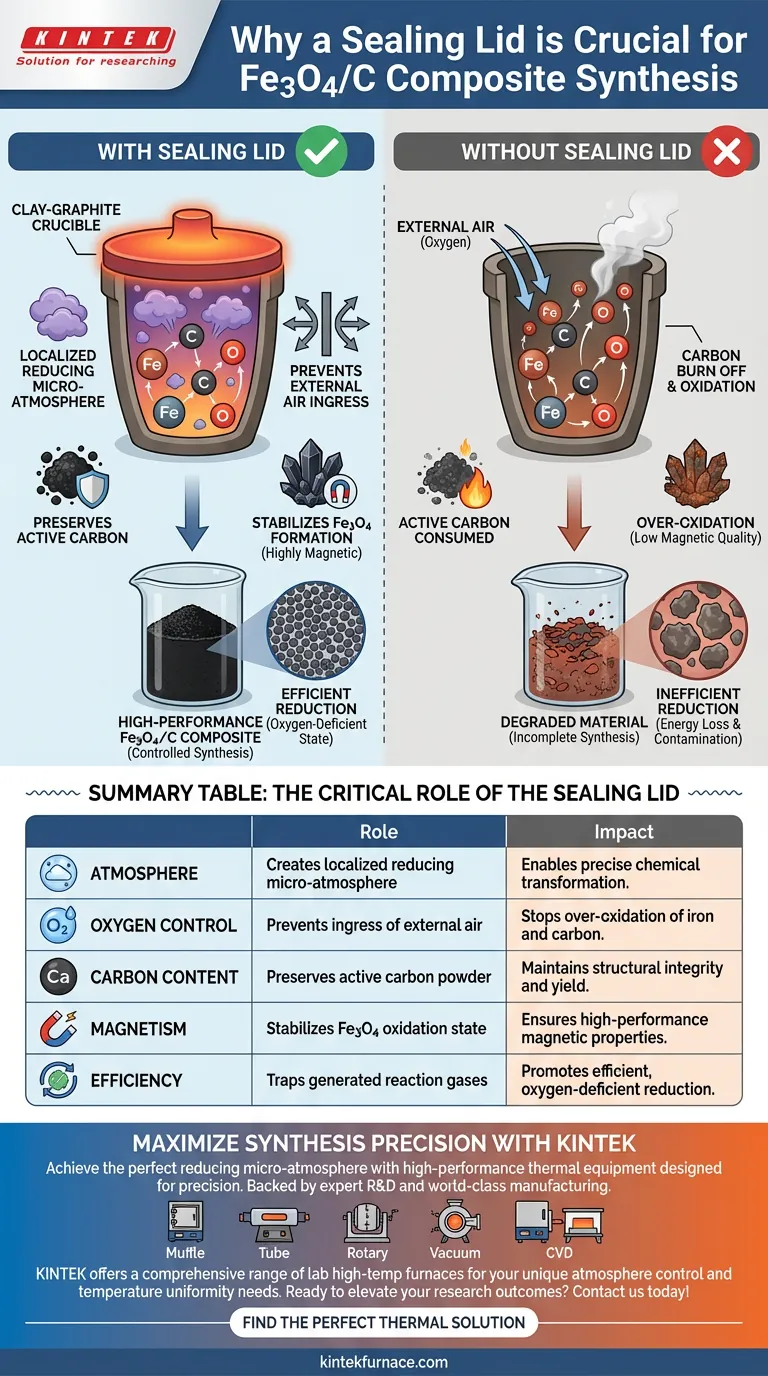
The primary function of the sealing lid is to physically isolate the reaction environment from the surrounding atmosphere. By preventing the ingress of external air, the lid allows the interior of the clay-graphite crucible to develop a localized reducing micro-atmosphere, which is critical for protecting the reactants and ensuring the correct chemical transformation occurs.
By sealing the crucible, you convert an open vessel into a controlled chemical reactor. This exclusion of oxygen protects the carbon and iron components from burning away or over-oxidizing, directly enabling the production of highly magnetic materials.

Creating a Controlled Chemical Environment
Establishing a Micro-Atmosphere
The synthesis of Fe3O4/C composites relies on specific chemical conditions that cannot exist in open air. The sealing lid traps the gases generated during heating, creating a localized reducing micro-atmosphere.
This internal environment dictates the direction of the chemical reaction. Without this containment, the reducing gases would escape, and the reaction equilibrium would shift unfavorably.
Preventing External Contamination
The most immediate role of the lid is to act as a physical barrier against external air.
If ambient oxygen is allowed to enter the crucible freely, it disrupts the sensitive reduction process. The lid ensures that the internal chemistry is driven by the reactants present, not by uncontrolled atmospheric variables.
Protecting Component Integrity
Preserving Active Carbon
The composite material relies heavily on active carbon powder as a structural and functional component.
In the presence of fresh oxygen at high temperatures, carbon is highly susceptible to oxidation (burning off). The sealing lid prevents this consumption, ensuring the carbon remains integrated into the final composite.
Stabilizing Fe3O4 Formation
The target material, Fe3O4 (magnetite), must be maintained in a specific oxidation state to retain its properties.
If the seal is missing, the newly formed Fe3O4 can be further oxidized by incoming air. This over-oxidation degrades the material quality and prevents the formation of the desired highly magnetic composite materials.
Understanding the Risks of Improper Sealing
The Consequence of Leakage
It is important to understand that a "partial" seal is often as detrimental as no seal.
If the lid does not fit tightly, the resulting draft can accelerate the oxidation of the carbon source. This leads to a lower yield and a composite with inconsistent magnetic properties.
Efficiency Losses
Without a proper seal, the reduction reaction becomes inefficient.
The system must work harder to overcome the presence of leaking oxygen, leading to wasted energy and potentially incomplete synthesis. A sealed environment ensures the reduction proceeds efficiently in an oxygen-deficient state.
Making the Right Choice for Your Goal
To ensure the successful synthesis of Fe3O4/C composites, the integrity of your crucible setup is paramount.
- If your primary focus is magnetic performance: Ensure the lid forms a tight seal to prevent oxidation of Fe3O4, which directly correlates to the material's magnetic strength.
- If your primary focus is material composition: Use a sealing lid to preserve the active carbon content, preventing it from burning away during the heating process.
The sealing lid is the single most critical variable in transitioning from a simple mixture of powders to a sophisticated, high-performance composite.
Summary Table:
| Feature | Role of Sealing Lid | Impact on Final Composite |
|---|---|---|
| Atmosphere | Creates localized reducing micro-atmosphere | Enables precise chemical transformation |
| Oxygen Control | Prevents ingress of external air | Stops over-oxidation of iron and carbon |
| Carbon Content | Preserves active carbon powder | Maintains structural integrity and yield |
| Magnetism | Stabilizes Fe3O4 oxidation state | Ensures high-performance magnetic properties |
| Efficiency | Traps generated reaction gases | Promotes efficient, oxygen-deficient reduction |
Maximize Your Material Synthesis Precision with KINTEK
Achieving the perfect reducing micro-atmosphere requires more than just a lid—it requires high-performance thermal equipment designed for precision. Backed by expert R&D and world-class manufacturing, KINTEK offers a comprehensive range of lab high-temp furnaces, including Muffle, Tube, Rotary, Vacuum, and CVD systems. Whether you are synthesizing Fe3O4/C composites or developing advanced ceramics, our systems are fully customizable to meet your unique atmosphere control and temperature uniformity needs.
Ready to elevate your research outcomes? Contact us today to find the perfect thermal solution for your lab!
Visual Guide
References
- Jiaxing Cai, Michael Hitch. Preparation of Fe3O4/C Composite Material from Red Mud for the Degradation of Acid Orange 7. DOI: 10.3390/ma18010151
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- Molybdenum Disilicide MoSi2 Thermal Heating Elements for Electric Furnace
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
People Also Ask
- What is the function of a vacuum rotary vane pump in hydrogen measurement? Ensure High-Purity Gas Analysis Baseline
- What are the thermal properties of alumina tubes? Discover Their High-Temp Durability and Stability
- How does the sealed Alumina Tube structure benefit the design of a reference electrode? Boost Electrolysis Precision
- What is the role of an optical pyrometer in diffusion bonding? Ensure Precision in High-Temperature Simulations
- What are the advantages of using a platinum crucible? Essential for High-Purity Alumino-Borosilicate Glass Synthesis
- What is the primary function of high-purity quartz sealed tubes? Master Sb-Te Alloy Synthesis with Precision Isolation
- Why is a ceramic crucible necessary for the thermal processing of silica extracted from sugarcane bagasse?
- What industries benefit from the use of alumina ceramic tubes? Essential for High-Temp, Corrosive Environments








