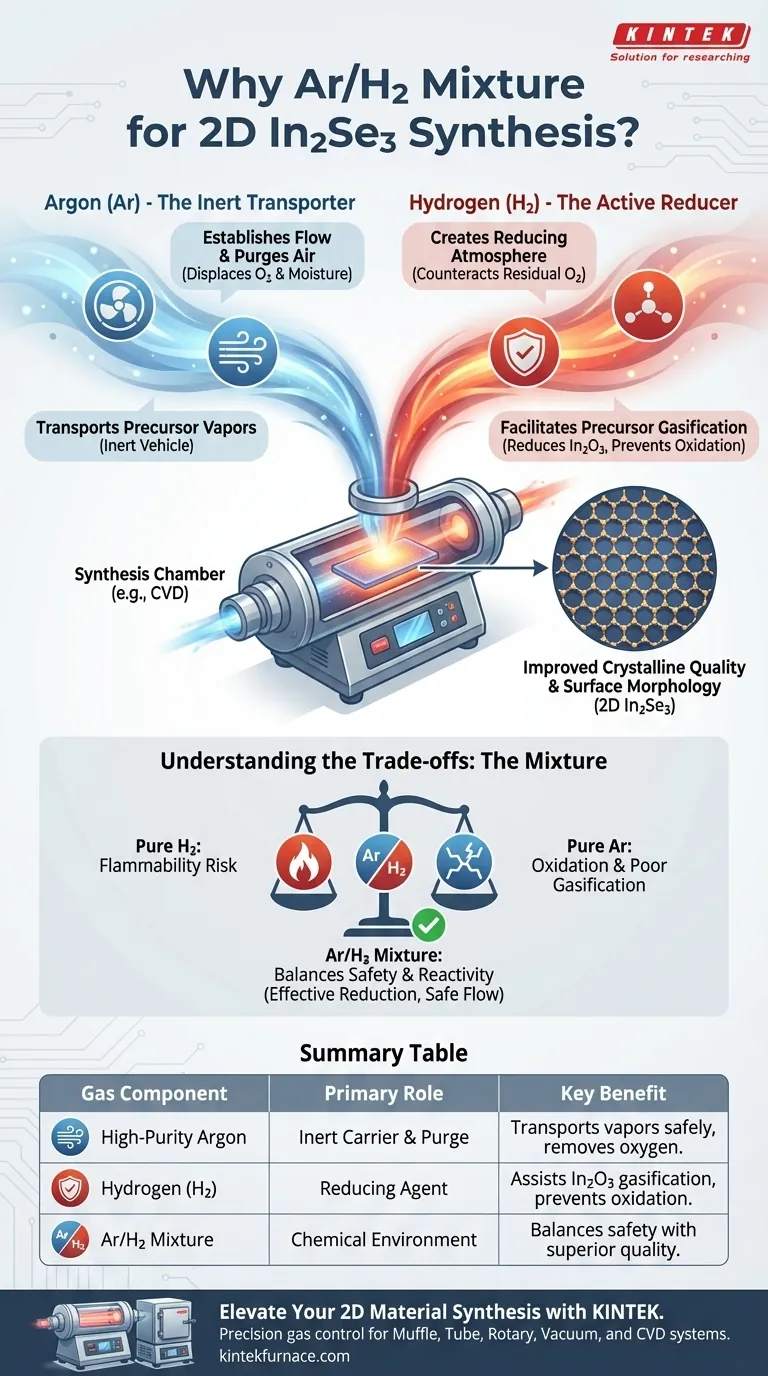
The use of a high-purity Argon and Hydrogen mixture is critical because it combines physical transport with chemical protection. While Argon serves as the inert vehicle to transport precursor vapors and purge the system of air, the addition of Hydrogen creates a necessary reducing atmosphere. This reducing environment actively assists in the gasification of the Indium Oxide (In2O3) precursor and prevents oxidation, directly leading to improved crystalline quality and surface morphology.
Core Insight: Argon provides the mechanical flow, but Hydrogen provides the chemical control. Without the specific reducing capabilities of Hydrogen, the precursor would not gasify efficiently, and the final 2D crystals would suffer from oxidation and poor structural integrity.

The Role of Argon: The Inert Transporter
Establishing the Flow
High-purity Argon acts as the primary carrier gas in this process. Its noble gas nature means it does not chemically react with the sensitive 2D materials, making it the ideal medium for physical transport.
Pre-Growth Purging
Before the heating process begins, Argon is responsible for purging air from the reaction chamber. By displacing atmospheric oxygen and moisture, it sets a baseline environment that prevents immediate contamination of the substrate and precursors.
Vapor Transport
During synthesis, Argon physically carries the generated precursor vapors from the source zone to the cooler deposition zone. This ensures a steady supply of material reaches the substrate for crystal growth.
The Role of Hydrogen: The Active Reducer
Creating a Reducing Atmosphere
While Argon is passive, Hydrogen is chemically active. The introduction of Hydrogen (typically in a 5% H2/Ar mixture) creates a reducing atmosphere. This is essential for counteracting any residual oxygen that the Argon purge might miss.
Facilitating Precursor Gasification
The primary reference highlights a specific chemical necessity: the reduction of the In2O3 precursor. Hydrogen assists in the reduction and subsequent gasification of Indium Oxide, ensuring that the Indium source is volatile enough to be transported to the substrate.
Enhancing Crystal Quality
Hydrogen does more than just protect against oxidation; it actively improves the final product. The presence of Hydrogen during growth regulates the surface chemistry, leading to improved crystalline quality and superior surface morphology of the 2D In2Se3 flakes.
Understanding the Trade-offs
The Necessity of a Mixture
You might ask why pure Hydrogen isn't used. Pure Hydrogen is highly flammable and poses safety risks. By using a mixture (e.g., 5% H2), you gain the chemical benefits of a reducing agent while maintaining the safety profile of an inert gas carrier.
Balancing Reactivity
The concentration of Hydrogen must be precise. It must be high enough to effectively reduce the In2O3 precursor and inhibit oxidation, but balanced with Argon to maintain the correct flow dynamics and partial pressures required for 2D vapor growth.
Making the Right Choice for Your Goal
To optimize your In2Se3 synthesis, consider these factors when configuring your gas flow:
- If your primary focus is Precursor Efficiency: Ensure your H2 concentration is sufficient (around 5%) to effectively reduce and gasify the In2O3 source, or you will see low yield.
- If your primary focus is Crystal Purity: Rely on the Argon purge cycle before growth, but depend on the continuous H2 flow to scavenge residual oxygen and prevent defects during the crystallization phase.
Summary: The Argon-Hydrogen mixture is not just a carrier; it is a tunable chemical tool that simultaneously transports material and engineers the atomic-level quality of your 2D crystals.
Summary Table:
| Gas Component | Primary Role | Key Benefit |
|---|---|---|
| High-Purity Argon | Inert Carrier & Purge | Transports vapors safely and removes atmospheric oxygen. |
| Hydrogen (H2) | Reducing Agent | Assists In2O3 gasification and prevents material oxidation. |
| Ar/H2 Mixture | Chemical Environment | Balances safety with superior crystalline morphology and purity. |
Elevate Your 2D Material Synthesis with KINTEK
Precision gas control and high-temperature stability are critical for growing superior 2D crystals like In2Se3. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems—all fully customizable to meet your specific research or production needs.
Don't let oxidation or poor gasification compromise your yields. Contact us today to discover how our advanced furnace technology can provide the perfect controlled environment for your lab's next breakthrough.
Visual Guide
References
- Dasun P. W. Guruge, Dmitri Golberg. Thermal Phase‐Modulation of Thickness‐Dependent CVD‐Grown 2D In<sub>2</sub>Se<sub>3</sub>. DOI: 10.1002/adfm.202514767
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- Split Chamber CVD Tube Furnace with Vacuum Station CVD Machine
- Multi Heating Zones CVD Tube Furnace Machine for Chemical Vapor Deposition Equipment
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- How does CVD compare to other coating methods like PVD? Uncover the Best Fit for Your Application
- What are the advantages of chemical vapor sterilization? Protect Your Metal Instruments from Rust and Dulling
- How does a Molecular Turbo Pump contribute to ZTO thin film quality? Master High-Vacuum Deposition Precision
- How does Chemical Vapor Deposition (CVD) differ from Physical Vapor Deposition (PVD)? Choose the Right Thin-Film Process
- Why are graphene nanoribbons considered potential candidates for digital devices? Unlock Next-Gen Electronics
- What is the purpose of a nested dual-quartz tube configuration in a CVD system? Optimize TB-MoS2 Synthesis Results
- Why must high vacuum and slow deposition rates be maintained for gold deposition? Unlock Precision Plasmonics
- MOCVD vs. PAMBE in beta-Ga2O3 Doping: Which System Is Best for Your Research?









