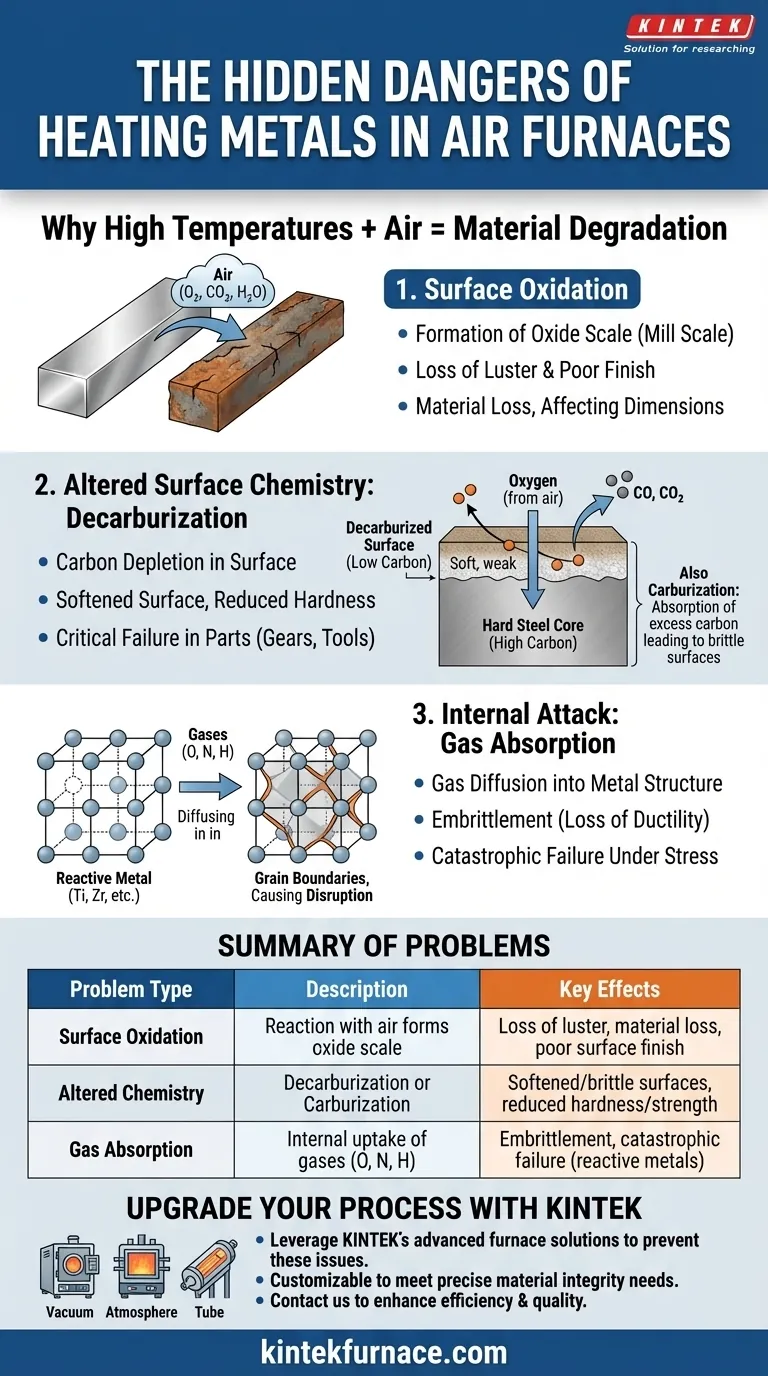
In short, heating ordinary metal materials in an air furnace introduces three primary problems: surface oxidation, changes in surface chemistry like decarburization, and the absorption of harmful gases. These uncontrolled reactions degrade the metal's surface finish, alter its mechanical properties, and can compromise the integrity of the final component.
The core issue is that at high temperatures, the seemingly harmless air in a furnace transforms into a chemically aggressive environment. This environment attacks the metal, stripping it of its desired characteristics and replacing them with flaws that can range from cosmetic to structurally critical.

The Primary Problem: Surface Oxidation
When a metal is heated, its atoms become more energetic and reactive. The oxygen, carbon dioxide, and water vapor present in the air readily react with the metal's surface.
How Oxidation Occurs
This chemical reaction forms a layer of metallic oxide on the part's surface. This layer, often called oxide scale or mill scale, is fundamentally different from the parent metal.
The Immediate Consequence: Oxide Scale
The most visible result is a loss of the metal's luster, replaced by a dull, often flaky or rough coating. This scale ruins the surface finish and can interfere with subsequent processes like painting, plating, or welding.
The Hidden Cost: Material Loss and Inaccuracy
The oxide scale is not just a coating; it is consumed parent material. This process leads to a loss of metal, which can be a significant problem for components that require precise dimensional tolerances.
The Secondary Threat: Altered Surface Chemistry
The gases in the air don't just react with the metal itself; they also react with key alloying elements within the metal, most notably carbon in steel.
Decarburization: The Loss of Hardness
Oxygen in the furnace atmosphere can react with the carbon near the surface of a steel part, forming carbon monoxide or carbon dioxide gas. This process, called decarburization, depletes the carbon content in the surface layer.
Since carbon is the primary element responsible for the hardness of steel, a decarburized surface becomes soft, losing its strength and wear resistance. This is a critical failure for parts like gears, bearings, or tools.
Carburization: An Unintended Addition
Conversely, if the furnace atmosphere is contaminated with carbon-rich gases like carbon monoxide or methane, the opposite effect can occur. The metal surface can absorb excess carbon, a process called carburization, leading to a brittle and unpredictable surface layer.
The Internal Attack: Gas Absorption
For certain chemically active metals, the problem goes deeper than the surface. At high temperatures, these metals can absorb gases directly into their internal structure.
For Chemically Active Metals
Metals like titanium, zirconium, and certain specialty alloys are highly susceptible to this issue. They have a strong affinity for gases like oxygen, nitrogen, and hydrogen.
How Gases Diffuse Inward
These gas atoms don't just stay on the surface. They diffuse into the metal, often settling along the grain boundaries of its crystalline structure.
The Result: Embrittlement and Failure
The presence of these interstitial gas atoms severely disrupts the metal's internal structure, causing a dramatic loss of ductility. This is known as embrittlement, and it can lead to premature and catastrophic failure of the component under stress.
Making the Right Choice for Your Process
Understanding these risks is crucial for selecting the appropriate heating method. The choice depends entirely on the material and the intended outcome of the heat treatment.
- If your primary focus is simple hot-working (e.g., forging): You may accept some oxidation and decarburization, as this damaged surface layer is often machined away in subsequent steps.
- If your primary focus is final heat treatment (e.g., hardening steel): An air furnace is often unsuitable, as decarburization will undermine the component's required surface hardness and wear resistance.
- If you are working with reactive metals (e.g., titanium): Using an air furnace is not an option. You must use a vacuum or inert gas furnace to prevent the catastrophic embrittlement caused by gas absorption.
Ultimately, controlling the furnace atmosphere is as critical as controlling the temperature when your goal is to achieve specific, reliable material properties.
Summary Table:
| Problem Type | Description | Key Effects |
|---|---|---|
| Surface Oxidation | Formation of oxide scale from reaction with air | Loss of luster, material loss, poor surface finish |
| Altered Surface Chemistry | Decarburization or carburization from gas reactions | Softened or brittle surfaces, reduced hardness and strength |
| Gas Absorption | Internal uptake of gases like oxygen and nitrogen | Embrittlement, catastrophic failure in reactive metals |
Upgrade your heat treatment process with KINTEK's advanced furnace solutions! Leveraging exceptional R&D and in-house manufacturing, we provide diverse laboratories with high-temperature furnaces like Muffle, Tube, Rotary, Vacuum & Atmosphere, and CVD/PECVD Systems. Our strong deep customization capability ensures precise solutions to prevent oxidation, decarburization, and embrittlement, meeting your unique experimental needs. Contact us today to enhance your material integrity and efficiency!
Visual Guide
Related Products
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
People Also Ask
- What are the development prospects of atmosphere box furnaces in the aerospace industry? Unlock Advanced Material Processing for Aerospace Innovation
- What are the primary inert gases used in vacuum furnaces? Optimize Your Heat Treatment Process
- What is an atmosphere protection muffle furnace? Unlock Precise Heat Treatment in Controlled Environments
- Can box type high-temperature resistance furnaces control the atmosphere? Unlock Precision in Material Processing
- What is inert gas technology used for in high-temperature atmosphere vacuum furnaces? Protect Materials and Speed Up Cooling



















