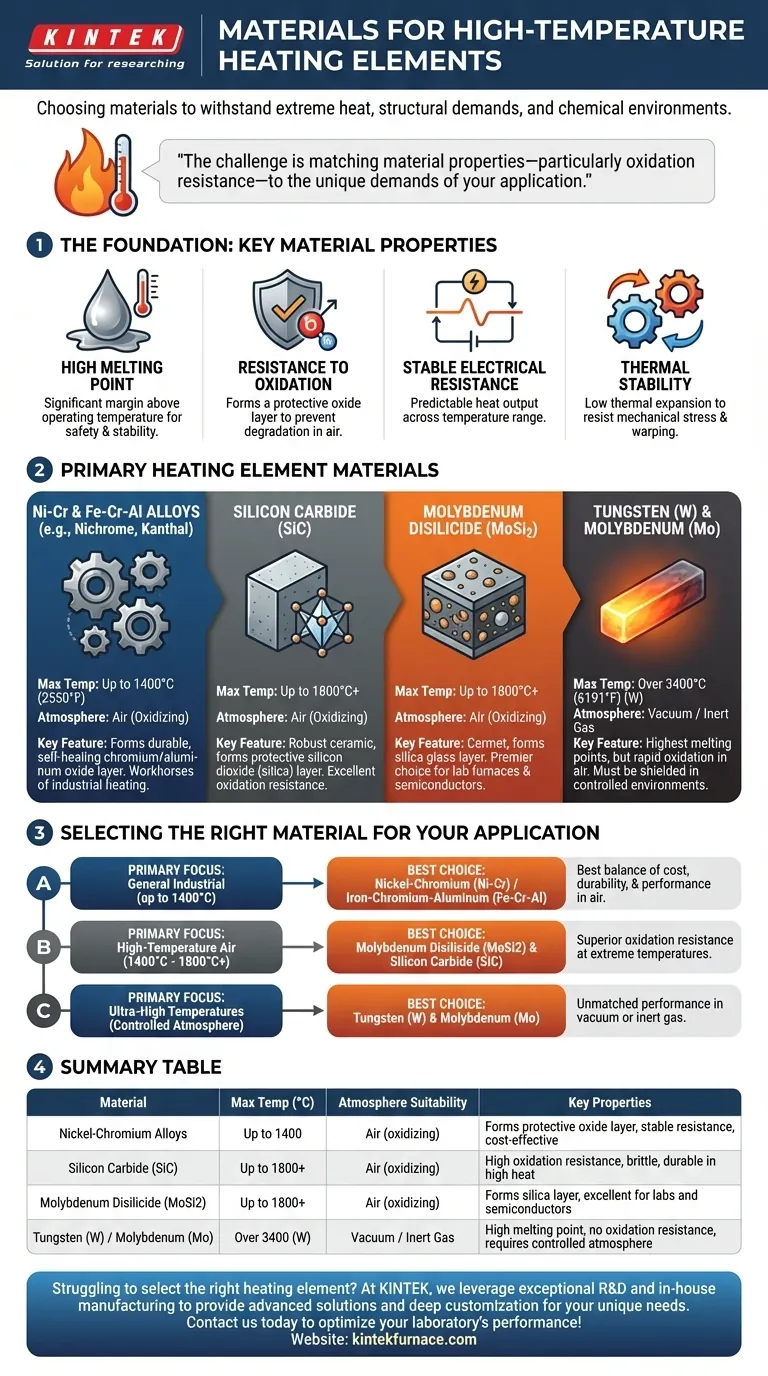
For high-temperature applications, the most common heating element materials are nickel-chromium alloys (like Nichrome), silicon carbide (SiC), molybdenum disilicide (MoSi2), and refractory metals like tungsten. These materials are chosen for their ability to withstand extreme heat while maintaining structural and electrical integrity. The final selection depends entirely on the required operating temperature, the chemical environment (i.e., air or vacuum), and cost constraints.
The challenge in selecting a heating element is not merely finding the material with the highest melting point. It's about matching the material’s specific properties—particularly its resistance to oxidation—to the unique demands and atmosphere of your application.
The Foundation of High-Temperature Heating: Key Properties
The performance of any heating element is dictated by a few fundamental material properties. Understanding these principles is the first step toward making an informed choice.
High Melting Point
The most obvious requirement is a melting point significantly higher than the intended operating temperature. This provides a crucial safety and operational margin.
Resistance to Oxidation
At high temperatures, most materials react with oxygen in the air, a process called oxidation. This degrades the material, causing it to fail. The best elements form a stable, protective oxide layer that prevents further corrosion.
Stable Electrical Resistance
A heating element works by converting electrical energy into heat through resistance. A material whose resistance remains relatively stable across a wide temperature range allows for predictable and controllable heat output.
Thermal Stability
Materials expand when heated. Elements with low thermal expansion are less prone to mechanical stress, warping, and fatigue during repeated heating and cooling cycles, leading to a longer service life.
A Breakdown of Primary Heating Element Materials
Each class of material offers a distinct profile of temperature limits, environmental compatibility, and physical characteristics.
Nickel-Chromium (Ni-Cr) and Iron-Chromium-Aluminum (Fe-Cr-Al) Alloys
These metallic alloys, known by trade names like Nichrome and Kanthal, are the workhorses of industrial and commercial heating. They are typically used in applications up to 1400°C (2550°F).
Their primary advantage is the formation of a durable, self-healing chromium oxide or aluminum oxide layer. This surface layer is highly resistant to oxidation, protecting the metal underneath even in open-air furnaces.
Silicon Carbide (SiC)
Silicon Carbide is a robust ceramic material capable of operating at higher temperatures than most metallic alloys in an air atmosphere.
When heated, SiC forms a protective layer of silicon dioxide (silica), which provides excellent oxidation resistance. It is often used in furnaces, kilns, and as an ignition source.
Molybdenum Disilicide (MoSi2)
As a ceramic-metal composite (cermet), molybdenum disilicide offers exceptional performance at very high temperatures, often exceeding 1800°C (3272°F) in oxidizing atmospheres.
Similar to SiC, it forms a protective silica glass layer on its surface when heated. This makes it a premier choice for laboratory furnaces and semiconductor processing where extreme, clean heat is required.
Tungsten (W) and Molybdenum (Mo)
These are refractory metals with the highest melting points of all materials on this list, with tungsten reaching over 3400°C (6191°F).
However, their critical weakness is a near-total lack of oxidation resistance. At high temperatures in the presence of air, they will rapidly burn away. Consequently, their use is strictly limited to vacuum furnaces or environments with an inert gas atmosphere.
Understanding the Trade-offs
Choosing a material is always a matter of balancing competing factors. The ideal material for one application may be completely unsuitable for another.
Atmosphere is Everything: Oxidation vs. Vacuum
This is the most critical trade-off. Ni-Cr alloys, SiC, and MoSi2 are designed to thrive in air because they form a protective oxide layer.
In contrast, tungsten and molybdenum must be shielded from oxygen. Using them in an air-filled furnace would lead to immediate failure.
Temperature Range vs. Cost
There is a direct correlation between maximum operating temperature and material cost. While Ni-Cr alloys are relatively economical, materials like MoSi2 and tungsten are significantly more expensive.
Pushing a material past its recommended temperature range is a false economy, as it dramatically shortens its lifespan and increases the risk of failure.
The Brittleness Factor
Metallic alloys like Nichrome are ductile and resistant to mechanical shock. Ceramic elements like SiC and MoSi2, however, are inherently brittle at room temperature and must be handled with care to avoid fracture.
Selecting the Right Material for Your Application
Use your primary goal to guide your decision.
- If your primary focus is general industrial furnaces up to 1400°C: Nickel-chromium (Ni-Cr) or iron-chromium-aluminum (Fe-Cr-Al) alloys offer the best balance of cost, durability, and performance in air.
- If your primary focus is high-temperature air furnaces (1400°C - 1800°C+): Molybdenum Disilicide (MoSi2) and Silicon Carbide (SiC) are the correct choices for their superior oxidation resistance at extreme temperatures.
- If your primary focus is ultra-high temperatures in a controlled atmosphere: Tungsten and Molybdenum are unmatched for their performance in vacuum or inert gas environments where oxidation is not a factor.
Ultimately, a successful design hinges on selecting the material that is engineered to survive its specific operating environment.
Summary Table:
| Material | Max Temperature (°C) | Atmosphere Suitability | Key Properties |
|---|---|---|---|
| Nickel-Chromium Alloys (e.g., Nichrome) | Up to 1400 | Air (oxidizing) | Forms protective oxide layer, stable resistance, cost-effective |
| Silicon Carbide (SiC) | Up to 1800+ | Air (oxidizing) | High oxidation resistance, brittle, durable in high heat |
| Molybdenum Disilicide (MoSi2) | Up to 1800+ | Air (oxidizing) | Forms silica layer, excellent for labs and semiconductors |
| Tungsten (W) / Molybdenum (Mo) | Over 3400 (W) | Vacuum / Inert Gas | High melting point, no oxidation resistance, requires controlled atmosphere |
Struggling to select the right heating element for your high-temperature needs? At KINTEK, we leverage exceptional R&D and in-house manufacturing to provide advanced solutions like Muffle, Tube, Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems. Our strong deep customization capability ensures we precisely meet your unique experimental requirements, enhancing efficiency and durability. Contact us today to discuss how our tailored heating elements can optimize your laboratory's performance!
Visual Guide
Related Products
- Molybdenum Vacuum Heat Treat Furnace
- Silicon Carbide SiC Thermal Heating Elements for Electric Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Laboratory Muffle Oven Furnace with Bottom Lifting
- Split Multi Heating Zone Rotary Tube Furnace Rotating Tube Furnace
People Also Ask
- What role does a high-temperature vacuum heat treatment furnace play in TBC post-processing? Enhance Coating Adhesion
- How does the ultra-low oxygen environment of vacuum sintering affect titanium composites? Unlock Advanced Phase Control
- What is the purpose of a 1400°C heat treatment for porous tungsten? Essential Steps for Structural Reinforcement
- What is the purpose of setting a mid-temperature dwell stage? Eliminate Defects in Vacuum Sintering
- Why is a vacuum environment essential for sintering Titanium? Ensure High Purity and Eliminate Brittleness



















