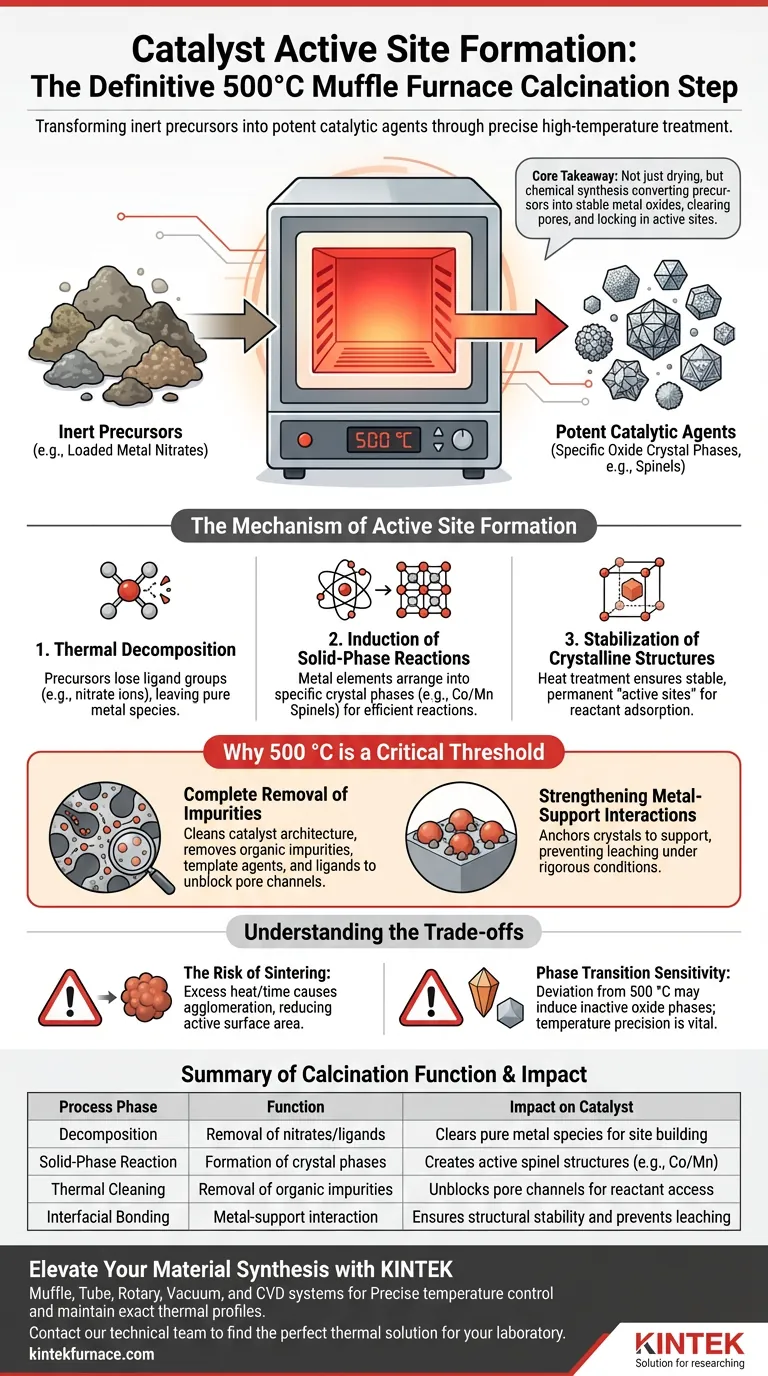
The muffle furnace calcination process at 500 °C is the definitive activation step that transforms inert precursor materials into potent catalytic agents. This high-temperature treatment drives the complete thermal decomposition of loaded metal nitrates, inducing solid-phase reactions that create specific, stable oxide crystal phases. These crystalline structures act as the core active sites required for efficient chemical reactions, such as toluene oxidation.
Core Takeaway Calcination at 500 °C is not merely a drying process; it is a chemical synthesis step that converts unstable precursors into defined metal oxide structures (like spinels). It simultaneously clears pore channels of impurities and "locks in" the active sites, ensuring the catalyst is both chemically active and structurally stable.
The Mechanism of Active Site Formation
Thermal Decomposition of Precursors
The primary function of the muffle furnace is to facilitate the breakdown of metal precursors, typically nitrates, that have been loaded onto a carrier.
At high temperatures, these precursors lose their ligand groups (such as nitrate ions).
This decomposition leaves behind the pure metal species required to build the active site.
Induction of Solid-Phase Reactions
Once the precursors decompose, the 500 °C environment induces solid-phase reactions between the metal elements.
This interaction is not random; it drives the elements to arrange themselves into specific crystal phases.
For example, this process can form spinel structures like (Co/Mn)(Co/Mn)2O4, which are highly effective for oxidation reactions.
Stabilization of Crystalline Structures
The heat treatment ensures that the resulting metal oxides are not amorphous or transient.
It promotes the formation of stable crystalline structures.
These stable crystals serve as the permanent "active sites" where reactants will later adsorb and react during industrial applications.
Why 500 °C is a Critical Threshold
Complete Removal of Impurities
Calcination at this specific temperature is essential for cleaning the catalyst's architecture.
It removes residual organic impurities, template agents, or ligands that might block the pore channels.
This "cleaning" ensures that the synthesized active sites are accessible to reactants rather than being buried under synthesis byproducts.
Strengthening Metal-Support Interactions
The uniform thermal field provided by a muffle furnace at 500 °C does more than just form crystals; it anchors them.
This temperature facilitates a strong interaction between the active metal oxides and the carrier material.
This bonding is critical for preventing the leaching or detachment of active species during rigorous reaction conditions.
Understanding the Trade-offs
The Risk of Sintering
While 500 °C is effective for crystallization, exceeding the optimal temperature or time can lead to sintering.
Sintering causes the small active particles to agglomerate into larger clumps, drastically reducing the active surface area.
This results in a catalyst that is chemically stable but physically inefficient due to fewer available active sites.
Phase Transition Sensitivity
Temperature precision is vital because catalyst performance often relies on a specific crystal phase.
Deviating significantly from the target temperature (e.g., 500 °C) may induce a transformation into an inactive oxide phase.
Therefore, the thermal stability provided by the muffle furnace is just as important as the absolute temperature.
Making the Right Choice for Your Goal
To optimize your catalyst preparation, tailor the calcination strategy to your specific performance metrics:
- If your primary focus is catalytic activity: Ensure your temperature program reaches the threshold required to form specific crystal phases (like spinels) without overshooting into sintering ranges.
- If your primary focus is structural longevity: Utilize the 500 °C hold time to maximize the interaction between the metal and the support, which prevents leaching.
- If your primary focus is pore accessibility: Verify that the temperature is sufficient to fully combust any organic templates or surfactants used during the initial synthesis.
The success of your catalyst relies on viewing calcination not as a heat treatment, but as a precise chemical reaction that defines the geometry of your active sites.
Summary Table:
| Process Phase | Function | Impact on Catalyst |
|---|---|---|
| Decomposition | Removal of nitrates/ligands | Clears pure metal species for site building |
| Solid-Phase Reaction | Formation of crystal phases | Creates active spinel structures (e.g., Co/Mn) |
| Thermal Cleaning | Removal of organic impurities | Unblocks pore channels for reactant access |
| Interfacial Bonding | Metal-support interaction | Ensures structural stability and prevents leaching |
Elevate Your Material Synthesis with KINTEK
Precise temperature control is the difference between a high-performance catalyst and a failed batch. Backed by expert R&D and manufacturing, KINTEK offers high-precision Muffle, Tube, Rotary, Vacuum, and CVD systems designed to maintain the exact thermal profiles required for critical calcination processes.
Whether you need uniform heat distribution for spinel formation or customizable atmospheres for sensitive phase transitions, our lab furnaces are built to meet your unique research and industrial needs.
Ready to optimize your active site formation? Contact our technical team today to find the perfect thermal solution for your laboratory.
Visual Guide
References
- Xiaojian Wang, Hao Huang. Synergistic oxidation of toluene through bimetal/cordierite monolithic catalysts with ozone. DOI: 10.1038/s41598-024-58026-6
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What temperature range can an electric muffle furnace typically reach? Explore Key Ranges and Uses
- How are box type resistance furnaces used in metallic material R&D? Unlock Precise Heat Treatment and Alloy Development
- What are the potential disadvantages of muffle furnaces? Key Trade-offs for Lab Precision
- What role does a high-temperature muffle furnace play in g-C3N4 catalyst synthesis? Precision Pyrolysis Solutions
- How do muffle furnaces ensure temperature uniformity? Discover Key Design Principles for Precise Heating
- How does a high-temperature muffle furnace convert shell powder to CaO? Achieve High-Purity Calcium Oxide via Calcination
- What is a muffle furnace and how is it related to laboratory furnaces? Discover Precision Heating for Your Lab
- What is the function of an industrial muffle furnace in g-C3N4 synthesis? Optimize Your Thermal Polymerization



















