At its core, the significance of good electrical conductivity in graphite is that it enables the very process of resistive heating. This property allows a controlled electrical current to flow through the element, and the material's inherent resistance converts this electrical energy directly into thermal energy with high efficiency.
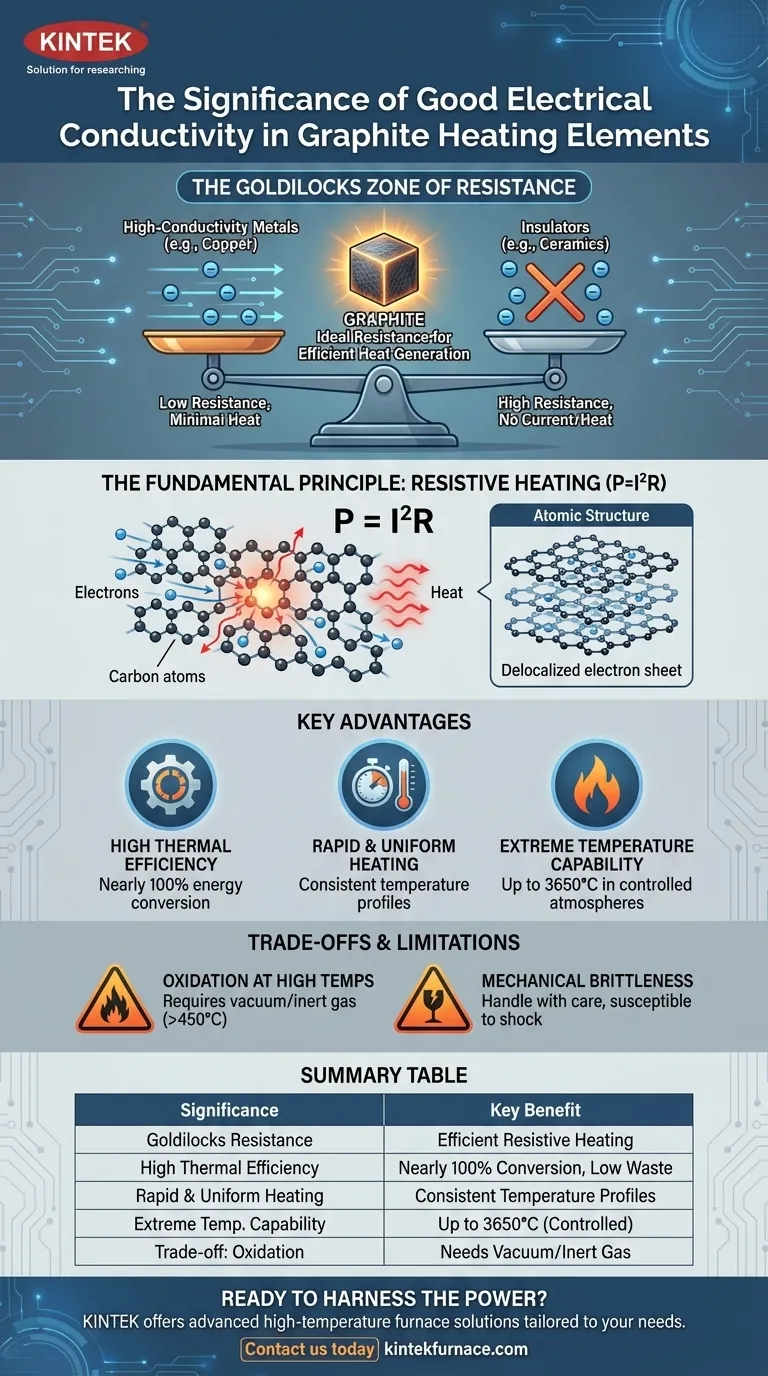
The crucial insight is that graphite's conductivity is not "good" in the way copper's is. Instead, it occupies a "Goldilocks zone"—conductive enough to carry current efficiently but resistive enough to generate substantial heat, a balance that makes it an exceptional material for high-temperature heating elements.
The Fundamental Principle: How Conductivity Creates Heat
To understand the significance of graphite's conductivity, we must first look at the principle of resistive heating, also known as Joule heating.
The Role of Electrical Resistance
When voltage is applied across a material, it forces electrons to flow, creating an electrical current. As these electrons travel, they collide with the atoms of the material.
Each collision transfers kinetic energy from the electron to the atomic lattice, causing the atoms to vibrate more intensely. This increased vibration is what we perceive as heat.
The property that governs this energy conversion is electrical resistance (R). The power (P) dissipated as heat is defined by the formula P = I²R, where I is the current.
Graphite's "Goldilocks" Conductivity
Materials are not simply "conductive" or "non-conductive"; they exist on a spectrum.
- High-Conductivity Metals (e.g., Copper): Have very low resistance. They are excellent for transmitting electricity with minimal heat loss, making them poor choices for heating elements.
- Insulators (e.g., Ceramics, Glass): Have extremely high resistance. They block the flow of current almost completely, so no heating can occur.
- Graphite: Sits in an ideal middle ground. Its conductivity is high enough to allow a significant current to flow with a reasonable voltage, but its resistance is substantial enough to generate intense heat according to the P = I²R formula.
The Atomic Structure Behind the Property
Graphite is composed of carbon atoms arranged in stacked, two-dimensional layers (graphene sheets). Within these layers, electrons are "delocalized" and can move freely, which accounts for its electrical conductivity.
However, the weaker bonds between the layers create impedance, giving graphite the moderate resistance needed to be an effective heater.
Key Advantages in Practice
Graphite's specific level of conductivity translates directly into tangible performance benefits for heating applications, particularly in demanding industrial environments.
High Thermal Efficiency
Because the heat is generated directly within the element itself, the conversion of electrical energy to thermal energy is nearly 100% efficient. This minimizes energy waste and reduces operational costs.
Rapid and Uniform Heating
The ability to pass current throughout the entire body of a well-designed graphite element ensures that it heats up very quickly and uniformly across its surface. This is critical for processes requiring consistent temperature profiles.
Extreme Temperature Capability
While conductivity enables the heating, graphite's other properties make it a star performer. It has an exceptionally high melting point (sublimating at ~3650°C) and actually gets stronger as temperature increases (up to ~2500°C). Its conductivity allows it to reach these extreme temperatures efficiently.
Understanding the Trade-offs and Limitations
No material is perfect. Acknowledging graphite's limitations is essential for proper application and design.
Oxidation at High Temperatures
This is graphite's single greatest weakness. In the presence of oxygen, graphite will begin to rapidly oxidize (burn) at temperatures above 450-500°C.
Therefore, graphite heating elements must be operated in a vacuum or an inert gas atmosphere (like argon or nitrogen) to prevent their destruction.
Mechanical Brittleness
Unlike ductile metallic heating elements that can bend, graphite is a brittle ceramic-like material. It is susceptible to fracture from mechanical shock or improper support and must be handled and installed with care.
The Impact of Purity and Grade
The electrical conductivity and performance of a graphite element are highly dependent on its purity, density, and grain structure. Different grades of graphite are manufactured for different purposes, and using the wrong grade can lead to unpredictable heating, hot spots, or premature failure.
Making the Right Choice for Your Application
Selecting a heating element requires matching the material's properties to the operational environment and performance goals.
- If your primary focus is high-temperature furnaces (vacuum or inert): Graphite is the superior choice due to its unparalleled temperature capability and efficiency in these non-oxidizing environments.
- If your primary focus is heating in open air: A metallic alloy element like Kanthal (FeCrAl) or Nichrome (NiCr) is the correct choice, as they form a protective oxide layer that prevents burnout.
- If your primary focus is extreme mechanical durability: Consider robust metallic elements or silicon carbide (SiC), which can offer greater resistance to mechanical shock than graphite.
Ultimately, understanding that graphite's electrical conductivity is a precisely balanced property is the key to leveraging it for powerful and efficient thermal systems.
Summary Table:
| Significance of Graphite's Conductivity | Key Benefit |
|---|---|
| Goldilocks Zone of Resistance | Enables efficient resistive heating, balancing current flow and heat generation |
| High Thermal Efficiency | Converts nearly 100% of electrical energy into heat, minimizing waste |
| Rapid & Uniform Heating | Allows current to flow throughout the element for consistent temperature profiles |
| Extreme Temperature Capability | Supports operation up to 3650°C in controlled atmospheres |
| Trade-off: Oxidation | Requires operation in a vacuum or inert gas atmosphere to prevent burnout |
Ready to harness the power of graphite heating elements in your lab?
At KINTEK, we leverage our exceptional R&D and in-house manufacturing to provide advanced high-temperature furnace solutions tailored to your unique experimental needs. Our product line, including Muffle, Tube, Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems, is complemented by strong deep customization capabilities.
Whether you require precise temperature control, uniform heating, or operation in extreme environments, our expertise in graphite heating technology ensures optimal performance and efficiency for your specific application.
Contact us today to discuss how our custom furnace solutions can elevate your research and development processes!
Visual Guide
Related Products
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
- Silicon Carbide SiC Thermal Heating Elements for Electric Furnace
- Laboratory Muffle Oven Furnace with Bottom Lifting
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Split Multi Heating Zone Rotary Tube Furnace Rotating Tube Furnace
People Also Ask
- Why is graphite a preferred material for heating elements in high-temperature vacuum furnaces?
- What is the mechanism and effect of post-annealing NiTi thin films in a vacuum furnace? Unlock Superelasticity
- What is the primary application of vacuum heat treating furnaces in aerospace? Enhance Component Performance with Precision
- How does vacuum heat treatment reduce workpiece deformation? Achieve Superior Dimensional Stability
- Why are graphite fixtures and holders important in vacuum furnaces? Unlock Precision & Durability












