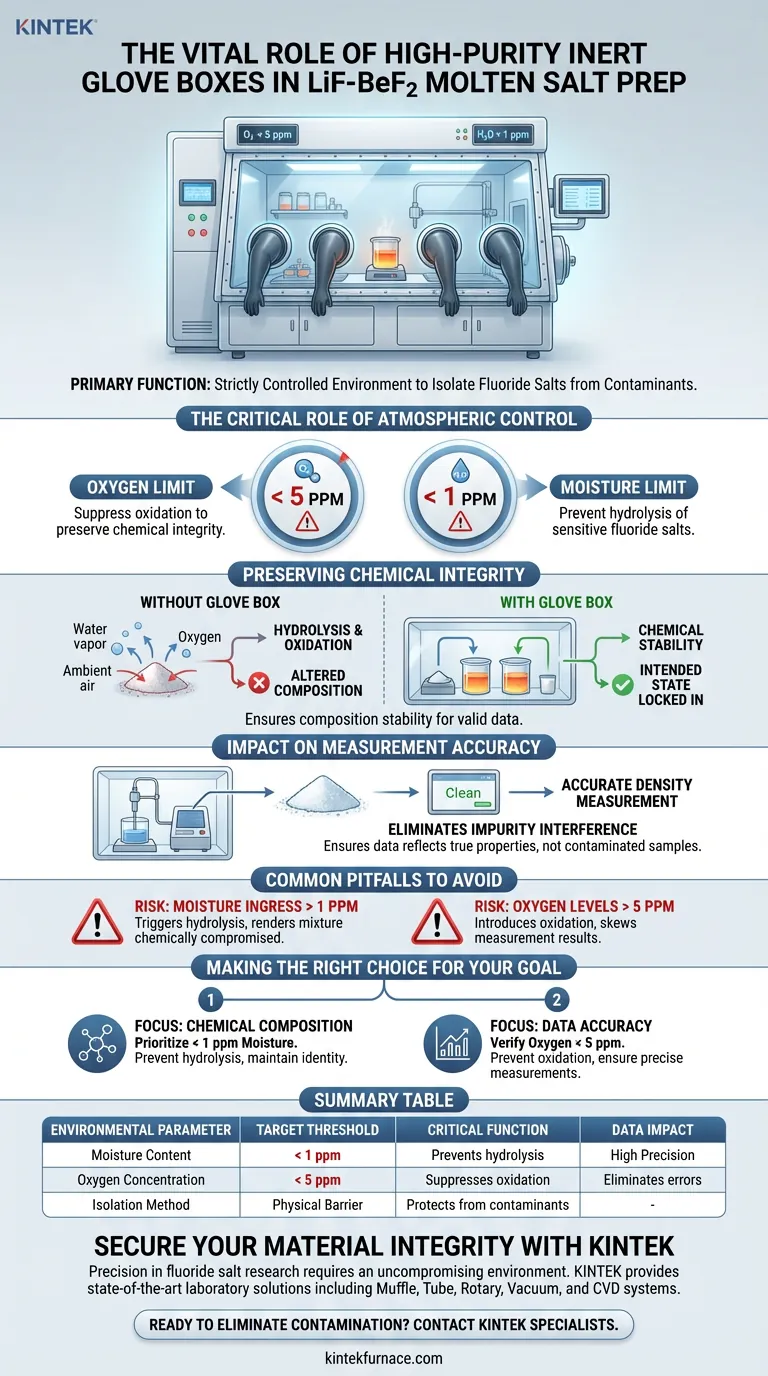
The primary function of a high-purity inert glove box is to enforce a strictly controlled environment where oxygen concentrations are kept below 5 ppm and moisture levels remain below 1 ppm. This equipment is utilized during the preparation of LiF-BeF2 molten salt systems to physically isolate the fluoride salts from ambient atmospheric contaminants.
By maintaining these ultra-low impurity levels, the glove box prevents the hydrolysis and oxidation of the salts, ensuring chemical stability and guaranteeing that subsequent density measurements are free from impurity-induced errors.

The Critical Role of Atmospheric Control
Defining the Purity Thresholds
The preparation of LiF-BeF2 mixtures requires an environment far cleaner than standard laboratory conditions. The glove box is specifically engineered to suppress oxygen to less than 5 parts per million (ppm).
Simultaneously, it must control moisture levels to less than 1 ppm. These specific thresholds are the operational baseline required to handle these sensitive materials safely and effectively.
Preserving Chemical Integrity
Preventing Hydrolysis and Oxidation
The fundamental purpose of this isolation is to stop chemical degradation. Fluoride salts are highly reactive to the water vapor and oxygen found in normal air.
Without the protection of the glove box, these salts would undergo hydrolysis or oxidation. This reaction alters the fundamental chemical composition of the mixture before it can be utilized or studied.
Ensuring Composition Stability
By excluding reactive elements, the glove box locks in the intended chemical state of the LiF-BeF2 system. This stability is not just about safety; it is a prerequisite for generating valid experimental data.
Impact on Measurement Accuracy
Eliminating Impurity Interference
The ultimate goal of using the glove box often extends to downstream analysis, such as density measurements. If the salts are exposed to air, the resulting impurities will skew these physical property readings.
Therefore, the glove box acts as a quality assurance tool. It ensures that any density data collected reflects the true properties of the pure salt mixture, rather than the properties of a contaminated or oxidized sample.
Common Pitfalls to Avoid
The Risk of Moisture Ingress
The most significant operational risk is exceeding the 1 ppm moisture limit. Even trace amounts of water vapor above this threshold can trigger hydrolysis, rendering the salt mixture chemically compromised for high-precision work.
Neglecting Oxygen Levels
Similarly, allowing oxygen to rise above the 5 ppm limit introduces the risk of oxidation. This is a common pitfall in systems where the inert gas purge is insufficient or glove box integrity is breached, leading to immediate impurity interference in measurement results.
Making the Right Choice for Your Goal
To ensure the success of your molten salt preparation, prioritize strict environmental monitoring based on your specific objectives:
- If your primary focus is Chemical Composition: rigorous adherence to the < 1 ppm moisture limit is required to prevent hydrolysis and maintain the fundamental identity of the fluoride salts.
- If your primary focus is Data Accuracy: verify that oxygen levels remain below 5 ppm to prevent oxidation-induced impurities from skewing density measurements.
The glove box is not merely a storage container; it is an active preservation system essential for the validity of your physical property data.
Summary Table:
| Environmental Parameter | Target Threshold | Critical Function |
|---|---|---|
| Moisture Content | < 1 ppm | Prevents hydrolysis of sensitive fluoride salts |
| Oxygen Concentration | < 5 ppm | Suppresses oxidation to preserve chemical integrity |
| Isolation Method | Physical Barrier | Protects salts from ambient atmospheric contaminants |
| Data Impact | High Precision | Eliminates impurity-induced errors in density measurement |
Secure Your Material Integrity with KINTEK
Precision in fluoride salt research begins with an uncompromising environment. KINTEK provides state-of-the-art laboratory solutions tailored for the most sensitive applications.
Backed by expert R&D and manufacturing, KINTEK offers Muffle, Tube, Rotary, Vacuum, and CVD systems, along with specialized high-purity processing environments. Whether you are handling LiF-BeF2 systems or complex alloys, our high-temp furnaces and inert gas systems are fully customizable to meet your unique research needs.
Ready to eliminate contamination and ensure data accuracy? Contact our specialists today to discuss your custom laboratory requirements.
Visual Guide
References
- Jisue Moon, Theodore M. Besmann. Density Measurements of Molten LiF–BeF<sub>2</sub> and LiF–BeF<sub>2</sub>–LaF<sub>3</sub> Salt Mixtures by Neutron Radiography. DOI: 10.1021/acsomega.4c01446
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
People Also Ask
- What role does the addition of NaCl as a diluent play in the SHS of Titanium Diboride? Master Nano-Powder Synthesis
- Why is pre-calcination of CaO, Al2O3, and V2O5 necessary? Achieve Stoichiometric Accuracy in Slag Samples
- Why are different cooling methods compared for GFRP post-fire performance? Evaluate Thermal Shock & Safety Risks
- What advantages does a microwave sintering furnace offer for LLZTO? Speed and Performance Compared
- How does temperature control precision affect SC-NMNO crystal morphology? Master Thermal Fields for High-Quality Grains
- What is the function of an inert gas supply system in black liquor pyrolysis? Achieve Precise Atmospheric Control
- What are the primary technical advantages of using stainless steel for the construction of horizontal pyrolysis furnace bodies? Durability and Thermal Precision
- What is the importance of preheating the mold? Master Thermal Control for Aluminum Matrix Composites














