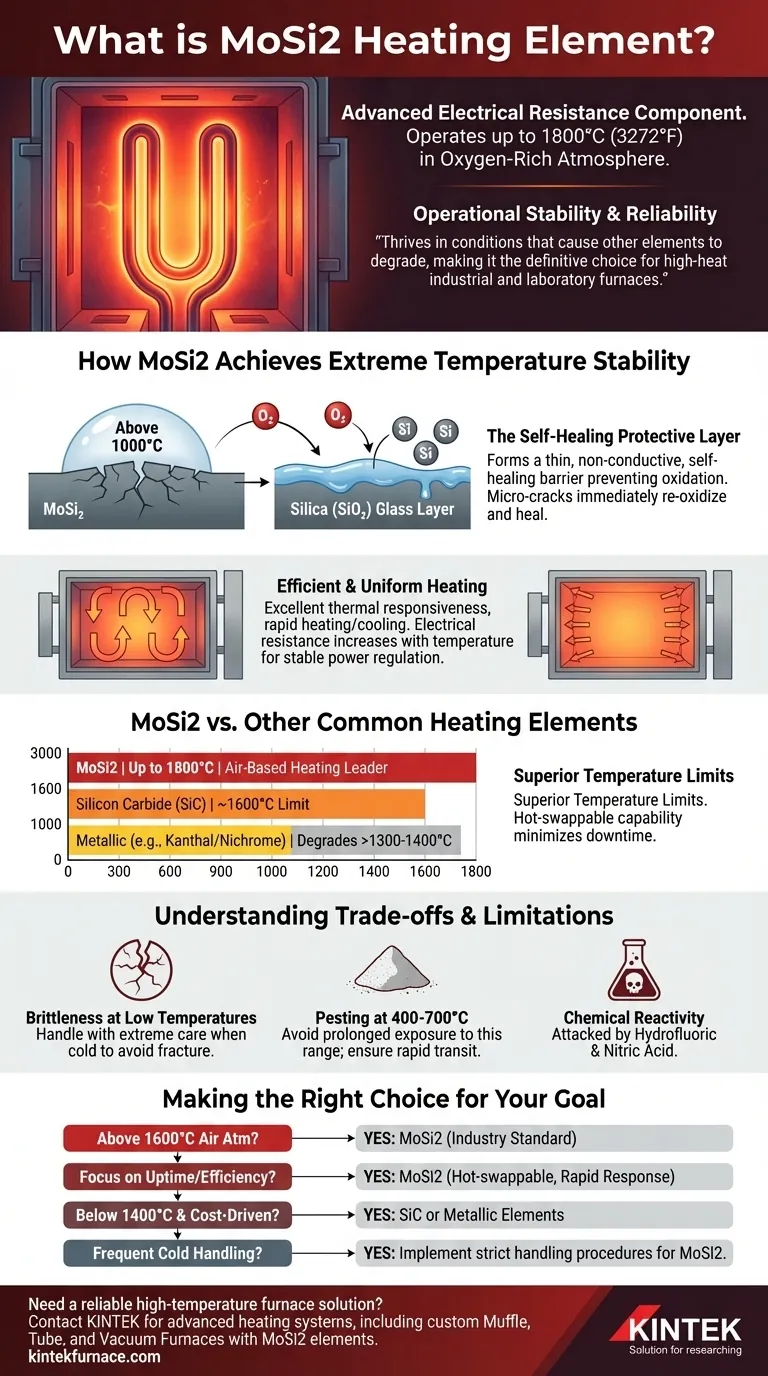
At its core, a MoSi2 heating element is an advanced electrical resistance component made from molybdenum disilicide. Its defining characteristic is the ability to operate at extremely high temperatures—up to 1800°C (3272°F)—in an oxygen-rich atmosphere, a feat that common metallic or silicon carbide elements cannot achieve. This performance is possible because the material forms a protective, self-healing glass layer on its surface when heated.
The true value of a MoSi2 element is not just its high-temperature capability, but its operational stability. It thrives in conditions that cause other elements to degrade, making it the definitive choice for high-heat industrial and laboratory furnaces where reliability and process consistency are paramount.

How MoSi2 Achieves Extreme Temperature Stability
The unique properties of MoSi2 are not inherent to the material at room temperature but are activated by the very heat it generates. This behavior is key to its success in demanding applications.
The Self-Healing Protective Layer
At temperatures above approximately 1000°C, the silicon within the MoSi2 element reacts with oxygen from the air. This reaction forms a thin, non-conductive, and viscous layer of pure silica (SiO₂), or quartz glass.
This silica layer acts as a protective barrier, preventing the underlying MoSi2 from further oxidation and degradation. If a micro-crack forms on this surface due to thermal stress, the exposed material immediately re-oxidizes, effectively "healing" the protective layer and ensuring long service life.
Efficient and Uniform Heating
MoSi2 elements possess excellent thermal responsiveness, allowing for rapid heating and cooling cycles. This significantly improves process efficiency in applications like sintering or heat treating.
Furthermore, their electrical resistance increases as they get hotter. This property helps regulate power and ensures stable, uniform temperature distribution across the furnace chamber, which is critical for consistent product quality.
MoSi2 vs. Other Common Heating Elements
Choosing a heating element requires understanding where MoSi2 fits in the broader landscape of high-temperature materials.
Superior Temperature Limits
MoSi2 elements are in a class of their own for air-based heating.
- MoSi2: Operates up to 1800°C.
- Silicon Carbide (SiC): Typically limited to around 1600°C.
- Kanthal (FeCrAl) & Nichrome: Metallic elements that generally degrade rapidly above 1300-1400°C.
Operational Advantages
Unlike many other element types, MoSi2 elements can often be replaced individually while the furnace is still hot. This "hot swapping" capability eliminates the need for a full cooldown and reheat cycle, preventing costly downtime in continuous production environments.
Understanding the Trade-offs and Limitations
No material is perfect. The exceptional high-temperature performance of MoSi2 comes with specific trade-offs that must be managed.
Brittleness at Low Temperatures
MoSi2 elements are ceramic-like and extremely brittle at room temperature. They must be handled with great care during shipping, installation, and any furnace maintenance performed when cold. Mechanical shock or stress can easily cause them to fracture.
"Pesting" at Intermediate Temperatures
In a specific temperature range, typically 400-700°C, MoSi2 can undergo a catastrophic form of accelerated oxidation known as "pesting." This process can cause the element to disintegrate into powder.
Well-designed furnaces and control systems mitigate this risk by ensuring the elements pass through this temperature zone quickly during both heat-up and cooldown.
Chemical Reactivity
While resistant to most acids and alkalis, MoSi2 will be attacked and dissolved by hydrofluoric acid and nitric acid. This is a critical consideration if your process involves these specific chemical compounds.
Making the Right Choice for Your Goal
Selecting the correct heating element depends entirely on your specific temperature, atmosphere, and operational requirements.
- If your primary focus is reaching temperatures above 1600°C in an air atmosphere: MoSi2 is the industry-standard solution due to its unique high-temperature oxidative resistance.
- If your primary focus is production uptime and process efficiency: The rapid thermal response and hot-swappable nature of MoSi2 elements offer significant operational advantages.
- If you are operating below 1400°C and cost is a primary driver: Traditional metallic elements like Kanthal (FeCrAl) or ceramic elements like SiC may offer a more economical solution.
- If your process requires frequent handling of cold components: You must implement strict, careful handling procedures for MoSi2 elements to prevent breakage due to their inherent brittleness.
By understanding these core principles, you are empowered to select and manage heating elements based on performance, longevity, and total cost of ownership.
Summary Table:
| Feature | MoSi2 Heating Element | Silicon Carbide (SiC) | Metallic (e.g., Kanthal) |
|---|---|---|---|
| Max Temp in Air | Up to 1800°C | ~1600°C | ~1300-1400°C |
| Key Advantage | Self-healing silica layer, hot-swappable | Good durability, lower cost | Cost-effective for lower temps |
| Limitation | Brittle when cold, avoid 400-700°C range | Lower max temp, slower response | Oxidizes rapidly above 1400°C |
Need a reliable high-temperature furnace solution for your lab? KINTEK leverages exceptional R&D and in-house manufacturing to provide advanced heating systems, including custom Muffle, Tube, and Vacuum Furnaces with MoSi2 elements. Our deep customization capabilities ensure your unique experimental requirements are met with precision and reliability. Contact us today to discuss how our high-temperature furnace solutions can enhance your process efficiency and stability!
Visual Guide
Related Products
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1400℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What is the function of a high-temperature muffle furnace in ZnO-SP preparation? Master Nanoscale Synthesis Control
- How does a high-temperature muffle furnace contribute to the thermal treatment process of chalcopyrite ore?
- Why is a high-temperature muffle furnace used for Ni-BN powder preheating? Achieve defect-free coating density.
- What is the function of a muffle furnace in LSCF modification? Achieve Precise Thermal Foundation for Advanced Ceramics
- How does high-temperature heating facilitate the conversion of rice husks into inorganic precursors for silica extraction?



















