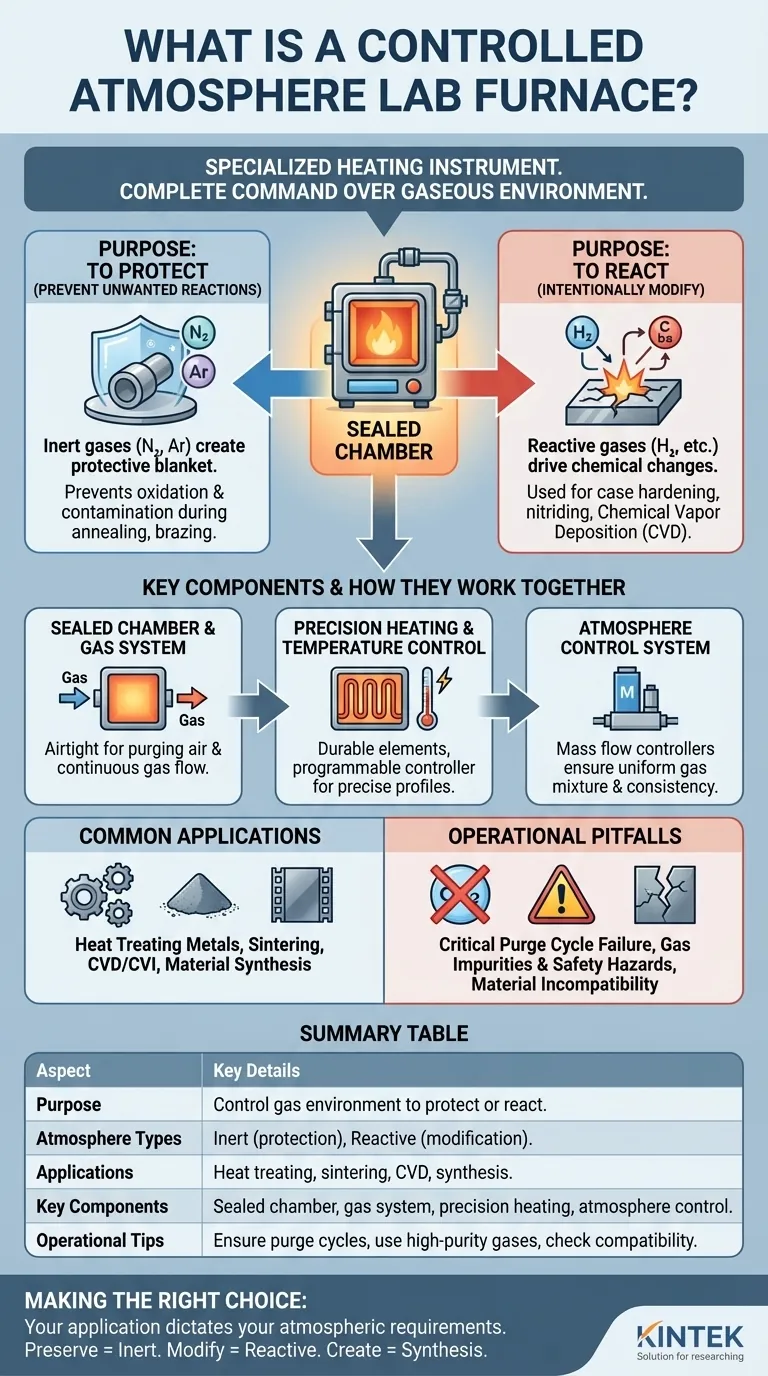
At its core, a controlled atmosphere furnace is a specialized heating instrument that gives you complete command over the gaseous environment surrounding your material. Unlike a standard furnace that operates in ambient air, this tool uses a tightly sealed chamber to introduce specific gases, allowing you to either protect a material from chemical change or intentionally cause a desired reaction at high temperatures.
The fundamental problem with heating materials in air is oxidation and contamination. A controlled atmosphere furnace solves this by replacing the air with a precisely managed gas environment, giving you the power to dictate the chemical outcome of your thermal process.

The Fundamental Purpose: Why Control the Atmosphere?
The "atmosphere" inside the furnace is the key to its function. The choice of gas defines whether the process will be protective or reactive, which is the most critical decision you will make.
To Protect: Preventing Unwanted Reactions
Many high-temperature processes, such as annealing or brazing, require the material to remain chemically unchanged. Exposing a metal to oxygen at high heat causes oxidation (rust), which can ruin its properties.
A controlled atmosphere furnace prevents this by purging the chamber of air and filling it with an inert gas, typically nitrogen (N2) or argon (Ar). These gases act as a protective blanket, ensuring the material's surface integrity is preserved.
To React: Intentionally Modifying a Material
In other cases, the goal is to purposefully change the material's surface chemistry. This is where reactive gases are used.
Gases like hydrogen (H2) can be used for reduction processes, while carbon-based gases can be used for carburizing to harden steel. Advanced techniques like Chemical Vapor Deposition (CVD) use reactive atmospheres to deposit entirely new layers of material onto a substrate.
Key Components and How They Work Together
A controlled atmosphere furnace is a system of integrated parts, each playing a critical role in achieving a stable and repeatable environment.
The Sealed Chamber and Gas System
The heart of the furnace is a high-temperature chamber engineered to be airtight. Gas inlet and outlet ports allow for the initial purging of oxygen and the continuous flow of the desired atmosphere throughout the heating cycle.
Precision Heating and Temperature Control
Durable heating elements, designed to withstand specific atmospheric conditions, provide the heat. This is governed by a sophisticated temperature control system, which uses thermocouples to measure the internal temperature and a programmable controller to execute precise heating and cooling profiles.
The Atmosphere Control System
This system is the brain of the gas environment. It uses mass flow controllers to precisely manage the flow rate and mixture of different gases. This ensures the atmospheric composition remains uniform and consistent, which is essential for achieving repeatable results.
Common Applications in Science and Industry
The ability to control the chemical environment at high temperatures unlocks a vast range of material processing capabilities.
Heat Treating Metals
This is one of the most common applications. Processes like annealing (softening), hardening, and tempering all rely on precise thermal cycles. Using a controlled atmosphere prevents surface scaling and ensures the desired metallurgical properties are achieved.
Sintering and Material Synthesis
Sintering is a process that fuses powders into a solid mass using heat. A controlled atmosphere is critical to prevent oxidation and promote the bonding between particles, which is essential for creating dense ceramics and metal parts.
Advanced Chemical Processing
Processes like Chemical Vapor Deposition (CVD) and Chemical Vapor Infiltration (CVI) are impossible in a standard furnace. They rely entirely on a reactive gas atmosphere to deposit thin films or infiltrate porous structures to create advanced composite materials.
Understanding the Operational Pitfalls
While powerful, these furnaces demand a disciplined operational approach. Overlooking key details can compromise your results and safety.
The Critical Purge Cycle
The most common mistake is failing to adequately purge the chamber of ambient air before starting the heating cycle. Any residual oxygen can cause unwanted oxidation, defeating the entire purpose of the furnace. A proper purge is non-negotiable.
Gas Purity and Safety
The quality of your source gas is paramount; impurities can act as contaminants. Furthermore, many process gases present safety hazards. Hydrogen is flammable and explosive, while nitrogen and argon are asphyxiants. Strict safety protocols and ventilation are essential.
Material and Gas Compatibility
Not all materials are compatible. Certain heating elements can be degraded by reactive gases at high temperatures. Likewise, the chamber insulation or refractory materials must be chosen to withstand the specific chemical environment of your process.
Making the Right Choice for Your Goal
Your application dictates your atmospheric requirements. The choice between an inert or reactive environment is the primary decision point.
- If your primary focus is preserving material integrity (e.g., annealing, brazing): You must use an inert atmosphere, like nitrogen or argon, to create a protective environment and prevent oxidation.
- If your primary focus is modifying a material's surface (e.g., case hardening, nitriding): You need a specific reactive atmosphere that is formulated to drive the desired chemical change on the material's surface.
- If your primary focus is creating new materials or coatings (e.g., CVD, sintering): Your choice of atmosphere is an active ingredient in the chemical synthesis, and its composition must be precisely controlled.
By mastering the atmosphere, you gain ultimate control over your material's final properties.
Summary Table:
| Aspect | Key Details |
|---|---|
| Purpose | Control gas environment to protect materials or cause reactions at high temperatures. |
| Atmosphere Types | Inert gases (e.g., nitrogen, argon) for protection; reactive gases (e.g., hydrogen) for modification. |
| Applications | Heat treating metals, sintering, CVD, and material synthesis. |
| Key Components | Sealed chamber, gas system, precision heating, atmosphere control. |
| Operational Tips | Ensure proper purge cycles, use high-purity gases, and check material compatibility. |
Ready to elevate your material processing with precision and control? KINTEK specializes in advanced high-temperature furnace solutions tailored to your unique needs. Leveraging exceptional R&D and in-house manufacturing, we offer Muffle, Tube, Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems, all with deep customization capabilities. Whether you're in research, industrial heat treatment, or material synthesis, our furnaces ensure reliable performance and repeatable results. Contact us today to discuss how we can support your laboratory goals!
Visual Guide
Related Products
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- Laboratory Muffle Oven Furnace with Bottom Lifting
People Also Ask
- What are some specific applications of atmosphere furnaces in the ceramics industry? Enhance Purity and Performance
- Can box type high-temperature resistance furnaces control the atmosphere? Unlock Precision in Material Processing
- What are the primary inert gases used in vacuum furnaces? Optimize Your Heat Treatment Process
- How does a mixed gas flow control system maintain stability during high-temperature nitriding? Precision Gas Ratios
- What is inert gas technology used for in high-temperature atmosphere vacuum furnaces? Protect Materials and Speed Up Cooling



















