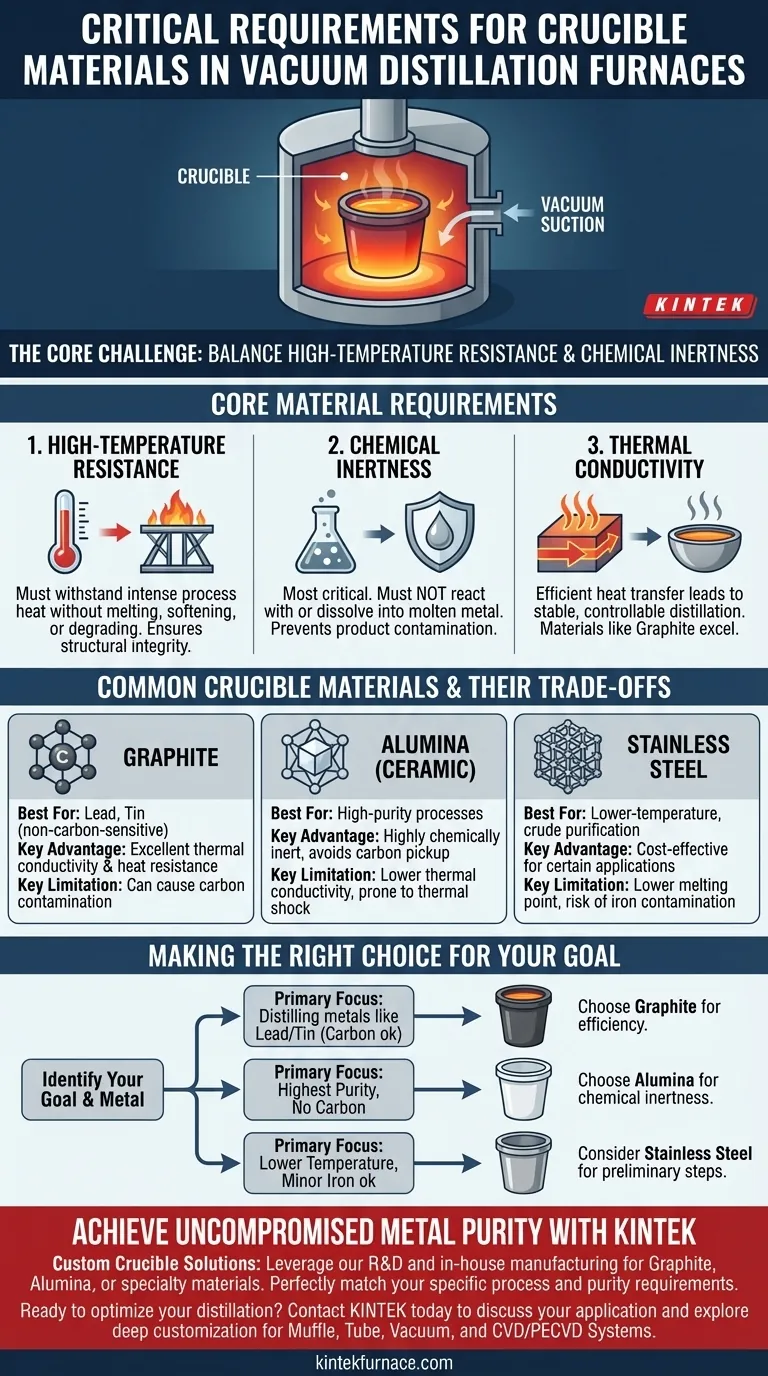
In short, the critical requirements for crucible materials in a vacuum distillation furnace are high-temperature resistance and chemical inertness. The chosen material must withstand the intense heat of the process without degrading while also remaining completely unreactive with the specific molten metal it contains to prevent contamination of the final product.
The core challenge is not just finding a material that can survive the heat, but selecting one that is chemically compatible with the metal being purified. The wrong choice can contaminate the very product you are trying to refine.

The Role of the Crucible in Vacuum Distillation
A vacuum distillation furnace refines metals by heating them until they vaporize and then condensing the pure metal vapor elsewhere, leaving impurities behind. The crucible is the vessel at the heart of this process.
The Crucible's Primary Function
The crucible's sole purpose is to hold the raw, molten metal inside the furnace chamber. It acts as a clean, stable container that can endure the extreme conditions required for vaporization.
Why the Vacuum Environment Matters
Operating under a vacuum lowers the boiling point of metals, allowing distillation to occur at more manageable temperatures. This environment, however, also means that any reaction between the crucible and the molten metal can proceed without interference from atmospheric gases, making material compatibility even more critical.
Core Material Requirements Explained
Choosing the right crucible material is a balancing act between thermal properties and chemical compatibility. The decision directly impacts the purity and quality of the distilled metal.
1. High-Temperature Resistance
The crucible must maintain its structural integrity at the specific operating temperature required to vaporize the metal. A material that melts, softens, or degrades under heat is entirely unsuitable.
2. Chemical Inertness
This is arguably the most crucial factor. The crucible must not react with, dissolve into, or otherwise contaminate the molten metal. Any reaction can introduce impurities, defeating the purpose of the distillation process.
3. Thermal Conductivity
Good thermal conductivity is highly desirable. It ensures that heat from the furnace is transferred efficiently and evenly to the metal charge, leading to a more stable and controllable distillation process. Materials like graphite excel in this area.
Common Crucible Materials and Their Trade-offs
The ideal crucible material is entirely dependent on the metal being processed. There is no single "best" option; each comes with specific advantages and disadvantages.
Graphite Crucibles
Graphite is a common choice due to its excellent heat resistance and superb thermal conductivity. It is frequently used for distilling metals like lead and tin.
- Key Limitation: Graphite is a source of carbon. It cannot be used when distilling metals that are sensitive to carbon contamination, as it can introduce carbon impurities into the final product.
Alumina (Corundum) Crucibles
Alumina is a ceramic material chosen specifically when carbon contamination is a primary concern. It is highly inert and can withstand very high temperatures.
- Key Limitation: Alumina crucibles generally have lower thermal conductivity than graphite, which can affect heating efficiency. They can also be more susceptible to thermal shock if heated or cooled too rapidly.
Stainless Steel Crucibles
Stainless steel is sometimes used in specific, less demanding scenarios. Its application is limited by its lower melting point compared to graphite or alumina.
- Key Limitation: It is only suitable for lower-temperature distillations or crude purification stages where potential iron contamination from the steel itself is an acceptable trade-off.
Making the Right Choice for Your Goal
Your selection must be guided by the specific chemical properties of the metal you are refining and the level of purity you need to achieve.
- If your primary focus is distilling metals like lead or tin where carbon is not a contaminant: Graphite is often the most efficient and cost-effective choice due to its excellent thermal properties.
- If your primary focus is achieving the highest purity and avoiding carbon contamination: An alumina crucible is the correct choice, as its chemical inertness will protect the integrity of the final product.
- If your primary focus is a lower-temperature process where minor iron contamination is acceptable: Stainless steel may be a viable option, particularly for preliminary refining steps.
Ultimately, the right crucible is the one that guarantees the purity of your specific metal under the required process conditions.
Summary Table:
| Material | Best For | Key Advantage | Key Limitation |
|---|---|---|---|
| Graphite | Lead, Tin (non-carbon-sensitive) | Excellent thermal conductivity & heat resistance | Can cause carbon contamination |
| Alumina (Ceramic) | High-purity processes | Highly chemically inert, avoids carbon pickup | Lower thermal conductivity, prone to thermal shock |
| Stainless Steel | Lower-temperature, crude purification | Cost-effective for certain applications | Lower melting point, risk of iron contamination |
Achieve Uncompromised Metal Purity with a Custom Crucible Solution
Selecting the right crucible is critical to the success of your vacuum distillation process. At KINTEK, we leverage our exceptional R&D and in-house manufacturing to provide advanced, custom high-temperature furnace solutions for diverse laboratories.
Our expertise in materials science ensures we can help you select or develop the ideal crucible—whether Graphite, Alumina, or a specialty material—to perfectly match your specific metal and purity requirements, preventing contamination and maximizing yield.
Ready to optimize your distillation process? Contact KINTEL today to discuss your application and discover how our deep customization capabilities for Muffle, Tube, Vacuum, and CVD/PECVD Systems can deliver the precision you need.
Visual Guide
Related Products
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- Laboratory Muffle Oven Furnace with Bottom Lifting
- Multi Zone Laboratory Quartz Tube Furnace Tubular Furnace
- 9MPa Air Pressure Vacuum Heat Treat and Sintering Furnace
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What role do tube furnaces play in semiconductor and battery production? Unlock Precision in High-Temp Processing
- What industrial and research applications are tube furnaces used for? Unlock Precise Thermal Processing Solutions
- What materials are used for the tubes in a High Temperature Tube Furnace? Choose the Right Tube for Your Lab
- What is the primary function of high-purity quartz sealed tubes? Master Sb-Te Alloy Synthesis with Precision Isolation
- What is the primary function of a vacuum-sealed quartz tube in MnBi2Te4 growth? Ensure High-Purity Crystal Synthesis



















