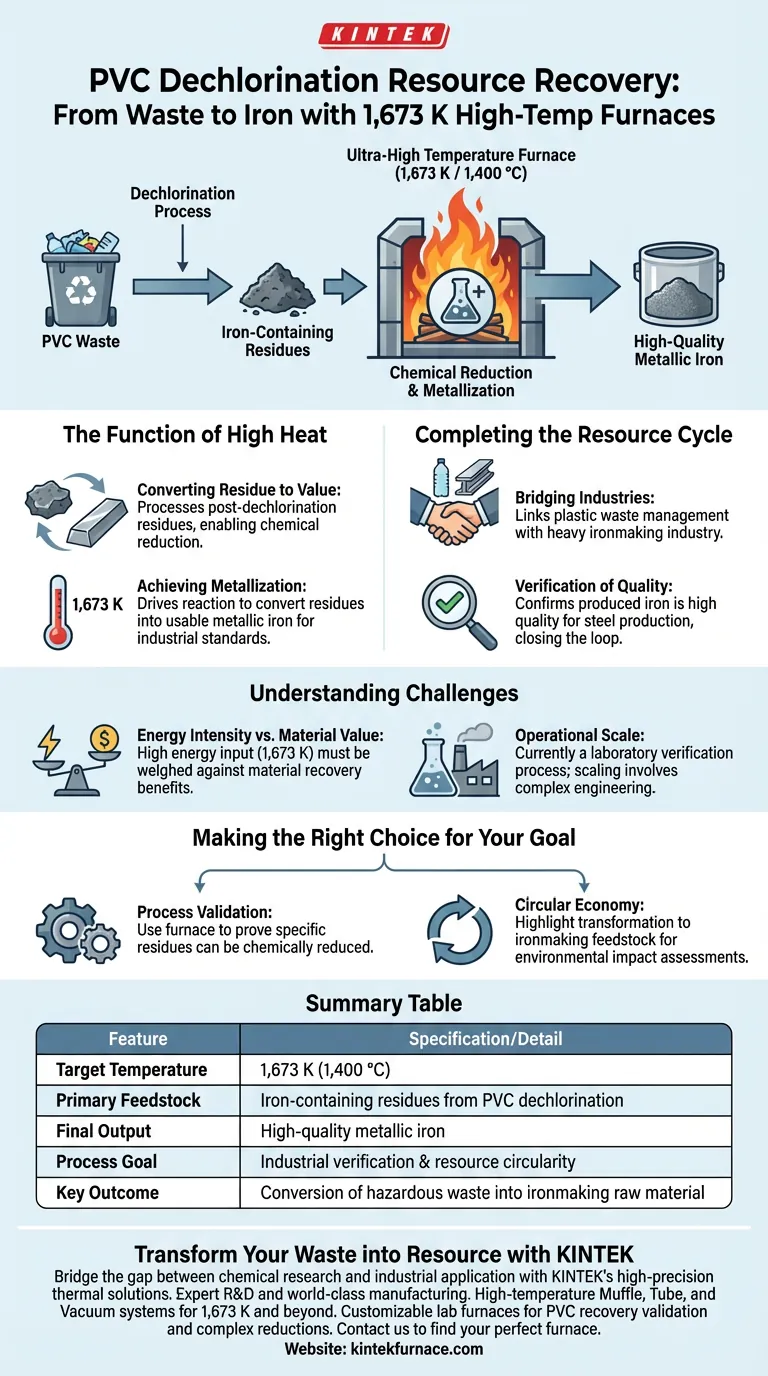
The ultra-high temperature furnace serves as a critical verification tool in the final stage of the PVC resource recovery cycle. Specifically, it functions to confirm the feasibility of converting iron-containing residues—which are byproducts left over after the dechlorination process—directly into usable metallic iron.
By subjecting post-dechlorination residues to extreme heat (1,673 K), this process demonstrates that hazardous plastic waste byproducts can be successfully transformed into high-quality raw materials for ironmaking, effectively closing the loop on resource utilization.

The Function of High Heat in Recovery
Converting Residue to Value
The primary purpose of the furnace is to process the iron-containing residues that remain after PVC has been dechlorinated.
Rather than treating this residue as secondary waste, the furnace creates an environment where chemical reduction can occur.
Achieving Metallization
Reaching a temperature of 1,673 K is necessary to drive the reaction that converts these chemical residues into metallic iron.
This high thermal threshold ensures the complete transformation of the material, verifying that the byproduct can meet the rigorous standards required for industrial application.
Completing the Resource Cycle
Bridging Plastic and Steel Industries
This application establishes a direct link between plastic waste management and heavy industry.
By validating that these residues can become feedstock for ironmaking, the process turns a disposal problem into a manufacturing solution.
Verification of Quality
The use of a laboratory furnace at this specific temperature is a proof-of-concept step.
It confirms that the iron produced from plastic waste residues is of sufficiently high quality to replace or supplement traditional raw materials in steel production.
Understanding the Challenges
Energy Intensity vs. Material Value
While the recovery of metallic iron is valuable, reaching 1,673 K requires significant energy input.
You must weigh the environmental benefit of material recovery against the energy cost of maintaining such high temperatures.
Operational Scale
The reference specifically notes the use of a laboratory furnace for verification.
This suggests that while the chemistry is sound, scaling this process to handle mass quantities of municipal PVC waste involves complex engineering logistics beyond the initial feasibility test.
Making the Right Choice for Your Goal
To determine how this technology fits into your resource recovery strategy, consider your primary objective:
- If your primary focus is process validation: Use the high-temperature furnace to prove that your specific dechlorination residues can be chemically reduced to metallic iron.
- If your primary focus is circular economy: Highlight the capability to transform plastic waste into ironmaking feedstock as a key metric for "closing the loop" in your environmental impact assessments.
This technology proves that with the right thermal treatment, even complex chemical residues can be reclaimed as valuable industrial assets.
Summary Table:
| Feature | Specification/Detail |
|---|---|
| Target Temperature | 1,673 K (1,400 °C) |
| Primary Feedstock | Iron-containing residues from PVC dechlorination |
| Final Output | High-quality metallic iron |
| Process Goal | Industrial verification & resource circularity |
| Key Outcome | Conversion of hazardous waste into ironmaking raw material |
Transform Your Waste into Resource with KINTEK
Bridge the gap between chemical research and industrial application with KINTEK’s high-precision thermal solutions. Backed by expert R&D and world-class manufacturing, KINTEK provides high-temperature Muffle, Tube, and Vacuum systems capable of reaching 1,673 K and beyond. Whether you are validating PVC recovery cycles or scaling complex chemical reductions, our customizable lab furnaces deliver the uniform heating and durability your project demands.
Ready to close the loop on your material recovery process? Contact us today to discuss your unique needs and find the perfect high-temp furnace for your laboratory.
Visual Guide
References
- Lan Hong, Lin-hai Ye. De-chlorination of poly(vinyl) chloride using Fe <sub>2</sub> O <sub>3</sub> and the improvement of chlorine fixing ratio in FeCl <sub>2</sub> by SiO <sub>2</sub> addition. DOI: 10.1515/htmp-2022-0299
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 600T Vacuum Induction Hot Press Vacuum Heat Treat and Sintering Furnace
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
People Also Ask
- What function does a High-Pressure Hydrogen Annealing Furnace serve? Achieving Deep Saturation in Steel Samples
- What are the main components of a vacuum furnace? Essential Parts for High-Temperature Processing
- What are the benefits of using a high-temperature vacuum furnace for the annealing of ZnSeO3 nanocrystals?
- Why use a vacuum diffusion annealing furnace for Zircaloy-4? Ensure Uniformity & Prevent Oxidation
- What are the advantages of using a vacuum heat treatment furnace? Optimize Fe-Mn-Si Alloy Solution Treatment
- Why must the drying process for MXene-coated electrodes be conducted in a vacuum drying oven? Key Stability Factors
- What are the specific requirements for the drying process in a vacuum drying oven? Essential MXene-ZrB2 Prep Steps
- What is the purpose of using a vacuum oven in hollow fiber membrane post-treatment? Ensure Structural Integrity



















