Ultimately, the heating element you choose defines the fundamental operational window of your sintering process. Its properties dictate the maximum achievable temperature, the furnace atmosphere you can use, the potential for product contamination, and the overall energy efficiency. Selecting the wrong element can lead to poor densification, unwanted chemical reactions, or premature equipment failure.
Choosing a heating element is not just a hardware decision; it is a strategic choice that sets the boundaries for your material's quality, your process's efficiency, and your operation's long-term cost. The ideal element must align perfectly with the thermal, chemical, and economic requirements of your specific sintering goal.

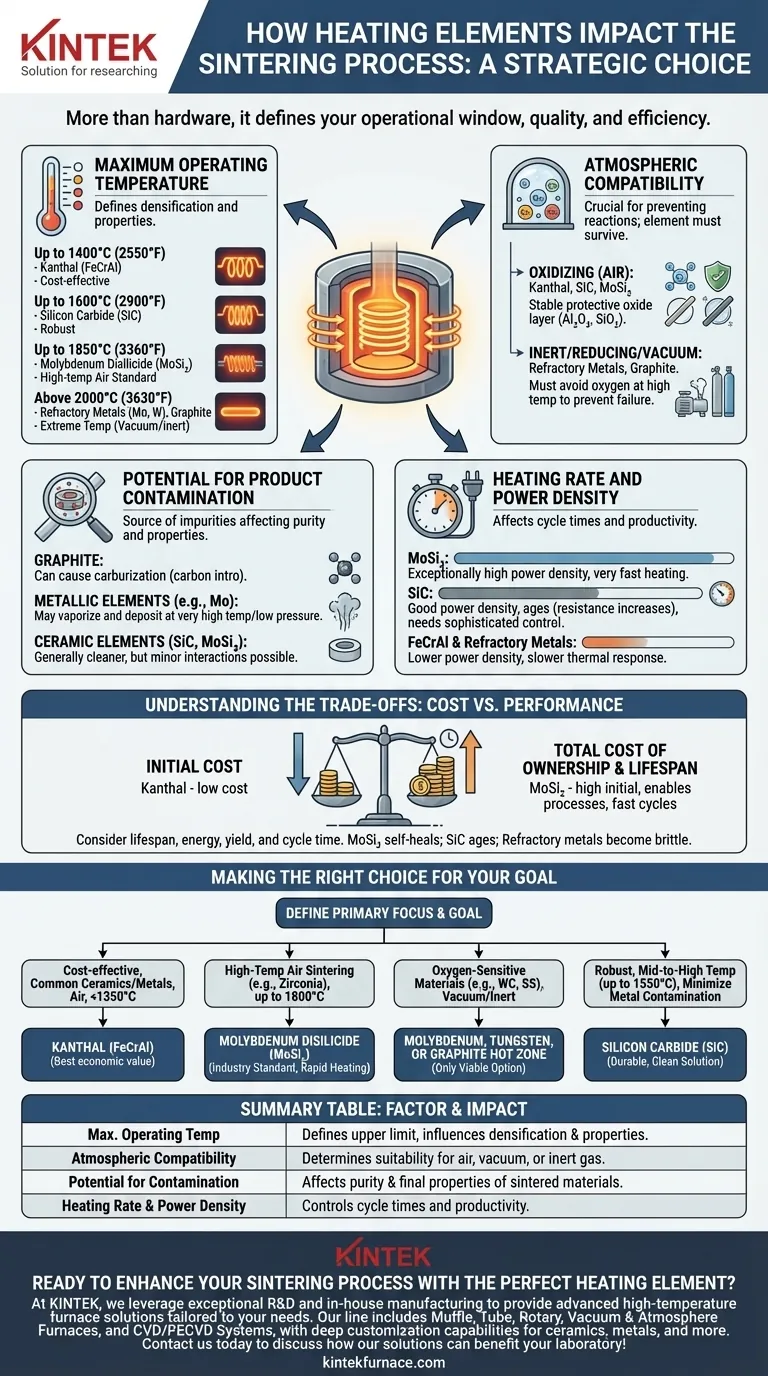
The Key Factors Dictated by Heating Elements
The impact of a heating element extends far beyond simply generating heat. Four primary factors are directly controlled by your selection, each critical to the success of the sintering cycle.
Maximum Operating Temperature
This is the most fundamental constraint. Sintering requires precise temperatures, often near the material's melting point, to drive diffusion and densification.
The element must be able to comfortably and reliably reach the target temperature without degrading. Common elements fall into distinct temperature classes.
- Up to 1400°C (2550°F): Kanthal (FeCrAl) alloys are the workhorse for lower-temperature applications. They are cost-effective and reliable in air.
- Up to 1600°C (2900°F): Silicon Carbide (SiC) elements offer a step up in temperature and are very robust.
- Up to 1850°C (3360°F): Molybdenum Disilicide (MoSi₂) is the standard for high-temperature air sintering, like that for zirconia or alumina ceramics.
- Above 2000°C (3630°F): Refractory metals (Molybdenum, Tungsten) and Graphite are used for extreme temperatures, but with a critical caveat.
Atmospheric Compatibility
The atmosphere inside the furnace is crucial for preventing unwanted chemical reactions, and the heating element must be able to survive in it.
An element that works perfectly in air can be destroyed in a vacuum or a reducing atmosphere, and vice-versa.
- Oxidizing Atmospheres (Air): Kanthal, SiC, and MoSi₂ are designed for this. They form a stable, protective oxide layer (Al₂O₃ or SiO₂) on their surface that prevents further oxidation.
- Inert/Reducing Atmospheres or Vacuum: Refractory metals (Molybdenum, Tungsten) and Graphite are required. If exposed to oxygen at high temperatures, they will oxidize catastrophically and fail almost instantly.
Potential for Product Contamination
The heating element itself can be a source of contamination, which can be detrimental to the purity and final properties of the sintered component.
This is especially critical when sintering high-purity ceramics, electronic materials, or medical-grade alloys.
- Graphite elements can cause carburization, introducing carbon into the product. This is unacceptable for many metals but can be desirable in specific carbide ceramic processes.
- Metallic elements (like Molybdenum) can, at very high temperatures and low pressures, vaporize slightly and deposit onto the part surface.
- Ceramic elements (SiC, MoSi₂) are generally "cleaner" but can still have minor interactions with highly sensitive materials.
Heating Rate and Power Density
The element's ability to convert electricity into heat quickly (power density) affects cycle times and productivity.
High power density allows for rapid heating ramps, shortening the overall sintering cycle. Low power density necessitates slower, more gradual heating.
- MoSi₂ elements have exceptionally high power density, enabling very fast heating rates.
- SiC offers good power density but its resistance increases over time (ages), requiring a more sophisticated power controller to maintain consistent output.
- FeCrAl and refractory metals generally have lower power densities, leading to slower thermal response.
Understanding the Trade-offs: Cost vs. Performance
Choosing an element is an exercise in balancing performance requirements with economic realities. The cheapest initial option is rarely the most cost-effective solution over the life of the furnace.
Initial Cost vs. Total Cost of Ownership
Kanthal (FeCrAl) is by far the least expensive element, but it is limited by temperature. MoSi₂ is one of the most expensive but enables processes that are otherwise impossible in air.
Consider the total cost, which includes element lifespan, energy consumption, and the impact on product yield and cycle time. An expensive element that allows for faster cycles and reduces scrap rates can provide a rapid return on investment.
Element Lifespan and Durability
Lifespan is not a fixed number; it is heavily dependent on operating temperature, atmosphere, and thermal cycling.
MoSi₂ is brittle at room temperature but becomes ductile at high temperatures and can "self-heal" damage to its protective silica layer.
SiC is mechanically robust but ages over its lifespan, requiring eventual replacement. Refractory metals like Molybdenum can become brittle after repeated high-temperature cycles (recrystallization), making them fragile during maintenance.
Making the Right Choice for Your Goal
To select the correct heating element, you must first define the non-negotiable requirements of your material and process. Your selection should flow directly from those needs.
- If your primary focus is cost-effective sintering of common ceramics or metals in air below 1350°C: Kanthal (FeCrAl) offers the best economic value.
- If your primary focus is high-temperature air sintering (e.g., zirconia dental crowns) up to 1800°C: Molybdenum Disilicide (MoSi₂) is the industry standard for its high temperature and rapid heating capabilities.
- If your primary focus is sintering oxygen-sensitive materials (e.g., tungsten carbide, stainless steel) in a vacuum or inert gas: A Molybdenum, Tungsten, or Graphite hot zone is your only viable option.
- If your primary focus is a robust, mid-to-high temperature process (up to 1550°C) where metallic contamination is a concern: Silicon Carbide (SiC) provides a durable and clean heating solution.
A systematic evaluation of these factors ensures your heating element is an asset to your process, not a limitation.
Summary Table:
| Factor | Impact on Sintering Process |
|---|---|
| Maximum Operating Temperature | Defines the upper limit for sintering, influencing densification and material properties. |
| Atmospheric Compatibility | Determines suitability for air, vacuum, or inert gas environments, preventing element failure. |
| Potential for Product Contamination | Affects purity and final properties of sintered materials, crucial for sensitive applications. |
| Heating Rate and Power Density | Controls cycle times and productivity through rapid or gradual heating capabilities. |
Ready to enhance your sintering process with the perfect heating element? At KINTEK, we leverage exceptional R&D and in-house manufacturing to provide advanced high-temperature furnace solutions tailored to your needs. Our product line includes Muffle, Tube, Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems, all backed by strong deep customization capabilities to precisely meet your unique experimental requirements. Whether you're working with ceramics, metals, or other materials, we can help you achieve superior results with optimal efficiency and cost-effectiveness. Contact us today to discuss how our solutions can benefit your laboratory!
Visual Guide
Related Products
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- Multi Zone Laboratory Quartz Tube Furnace Tubular Furnace
People Also Ask
- What is the role of a muffle furnace in the synthesis of water-soluble Sr3Al2O6? Precision in SAO Production
- What is the key role of a muffle furnace in the pretreatment of boron sludge and szaibelyite? Unlock Higher Process Efficiency
- What metals cannot be heated by induction? Understanding Material Suitability for Efficient Heating
- What is the role of a muffle furnace in the study of biochar regeneration and reuse? Unlock Sustainable Water Treatment
- What environmental conditions are critical for SiOC ceramicization? Master Precise Oxidation & Thermal Control



















