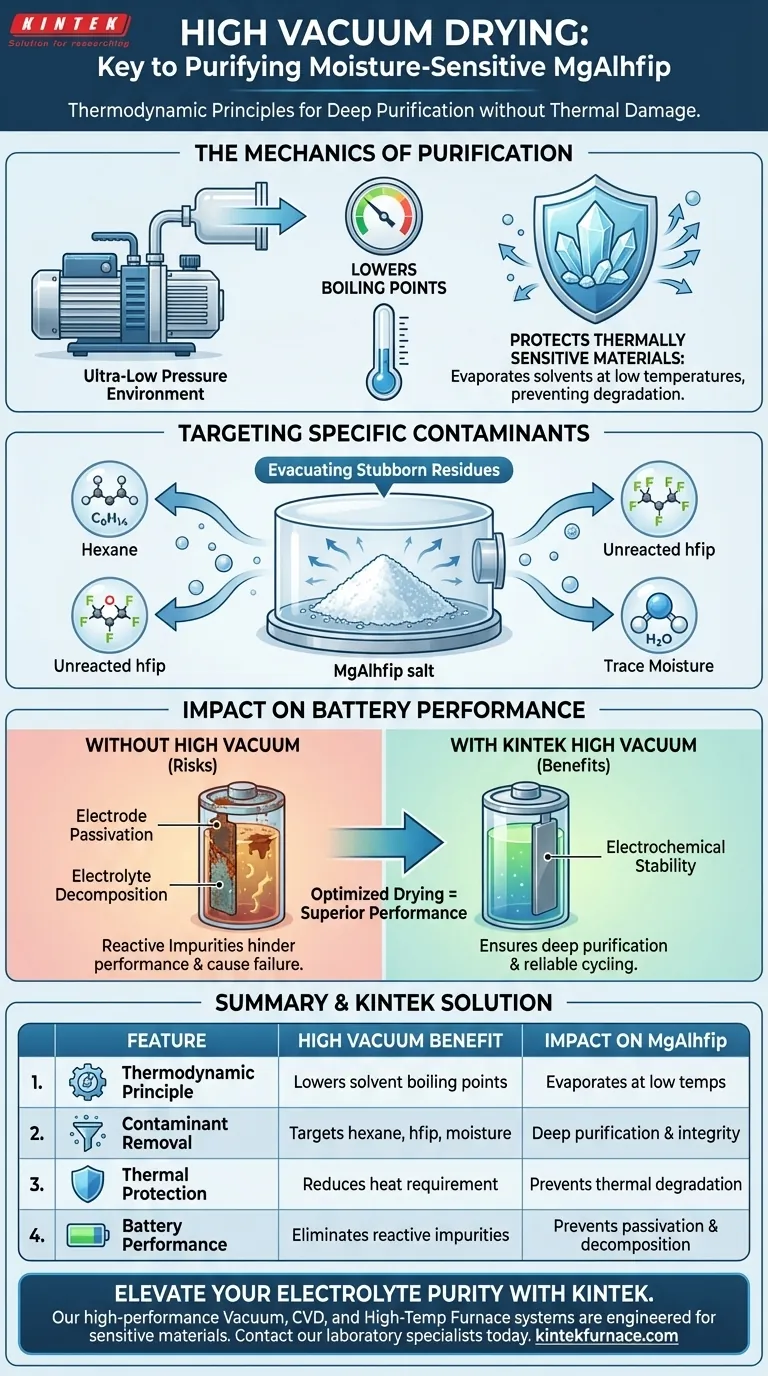
High vacuum drying operates on thermodynamic principles to drastically lower the boiling points of volatile residues within the salt. By creating an extremely low-pressure environment, the system facilitates the evaporation of stubborn solvents—specifically hexane, unreacted hfip, and trace moisture—without requiring excessive heat. This protects the moisture-sensitive MgAlhfip salt from thermal degradation while ensuring deep purification.
High vacuum drying is not merely about removing water; it is a critical purification step that safeguards electrochemical stability. By lowering the boiling point of solvents, it removes impurities without thermal damage, preventing electrode passivation and electrolyte decomposition.

The Mechanics of Purification
Manipulating Boiling Points
The primary mechanism relies on reducing the atmospheric pressure surrounding the salt. This significantly reduces the boiling points of residual solvents and organic matter trapped within the crystal structure.
Protecting Thermally Sensitive Materials
Because the boiling points are lowered, contaminants can be boiled off at much lower temperatures. This is vital for MgAlhfip, which is moisture-sensitive and potentially prone to thermal degradation if exposed to high heat.
Targeting Specific Contaminants
Removing Synthesis Byproducts
The synthesis of MgAlhfip often leaves behind volatile organic compounds. The high vacuum system specifically targets hexane and unreacted hfip (hexafluoroisopropanol), ensuring they are fully evacuated from the final product.
Eliminating Trace Moisture
Water is the enemy of magnesium electrolytes. The vacuum system removes even trace moisture that may have adhered to the salt during handling or synthesis.
Impact on Battery Performance
Preventing Electrode Passivation
If impurities like unreacted hfip or moisture remain, they react chemically within the battery cell. This leads to electrode passivation, where a non-conductive layer forms on the electrode surface, hindering performance.
Maintaining Electrochemical Stability
Thorough drying prevents the electrolyte from decomposing during operation. By removing the catalysts for decomposition (impurities), the vacuum process ensures the electrochemical stability of the salt is maintained.
Understanding the Risks of Inadequate Drying
The Cost of Residual Impurities
If the vacuum level is insufficient, volatile organics may remain trapped in the salt lattice. These residuals are not benign; they actively contribute to electrolyte decomposition once voltage is applied.
The Sensitivity Factor
MgAlhfip is highly sensitive to its environment. Failing to achieve a high enough vacuum forces operators to use higher temperatures to dry the salt, which risks damaging the salt's chemical structure before it ever enters a battery.
Making the Right Choice for Your Goal
To optimize the performance of magnesium electrolyte salts, consider these priorities:
- If your primary focus is Electrochemical Stability: Ensure the vacuum system is capable of removing "heavy" organic residues like unreacted hfip, not just surface water.
- If your primary focus is Cycle Life: Prioritize the complete removal of trace moisture to prevent passivation layers that degrade battery capacity over time.
A high vacuum system is the only reliable method to purify MgAlhfip without compromising its structural integrity.
Summary Table:
| Feature | High Vacuum Drying Benefit | Impact on MgAlhfip |
|---|---|---|
| Thermodynamic Principle | Lowers solvent boiling points | Evaporates residues at low temperatures |
| Contaminant Removal | Targets hexane, hfip, & moisture | Ensures deep purification & chemical integrity |
| Thermal Protection | Reduces heat requirement | Prevents thermal degradation of sensitive salts |
| Battery Performance | Eliminates reactive impurities | Prevents electrode passivation & decomposition |
Elevate Your Electrolyte Purity with KINTEK
Don't let residual moisture or solvents compromise your battery research. KINTEK’s high-performance vacuum systems are engineered to provide the precise low-pressure environments required for sensitive materials like MgAlhfip. Backed by expert R&D and world-class manufacturing, we offer customizable Vacuum, CVD, and High-Temp Furnace systems designed to protect your samples from thermal damage while ensuring maximum electrochemical stability.
Ready to optimize your drying process? Contact our laboratory specialists today to find the perfect system for your unique needs.
Visual Guide
References
- Andrijana Marojević, Jan Bitenc. Influence of Salt Concentration on the Electrochemical Performance of Magnesium Hexafluoroisopropoxy Aluminate Electrolyte. DOI: 10.1002/batt.202500497
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Ultra Vacuum Electrode Feedthrough Connector Flange Power Lead for High Precision Applications
- Magnesium Extraction and Purification Condensing Tube Furnace
- CF KF Flange Vacuum Electrode Feedthrough Lead Sealing Assembly for Vacuum Systems
- Ultra High Vacuum Stainless Steel KF ISO CF Flange Pipe Straight Pipe Tee Cross Fitting
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
People Also Ask
- What role do the vacuum arc furnace and titanium getter play in refractory medium-entropy alloy production? Mastering Purity & Power
- Why is a high-vacuum probe station necessary for SnS2 analysis? Ensure Pure Electrical Characterization
- Why is a vacuum drying oven required for lithium-sulfur battery electrodes? Ensure High-Purity Testing Results
- What industries commonly use vacuum brazing furnaces? Essential for Aerospace, Medical, Automotive, and Electronics
- What types of heat treatment processes use vacuum furnaces? Achieve Purity and Precision in Material Processing
- What industries benefit from using vacuum furnaces? Achieve Purity and Precision in High-Stakes Sectors
- Why is a molybdenum-lined furnace preferred for sintering MIM steel parts? Prevent Carbon Contamination
- What feature of vacuum furnaces makes them suitable for large-scale manufacturing? Unmatched Scalability & Reproducibility



















