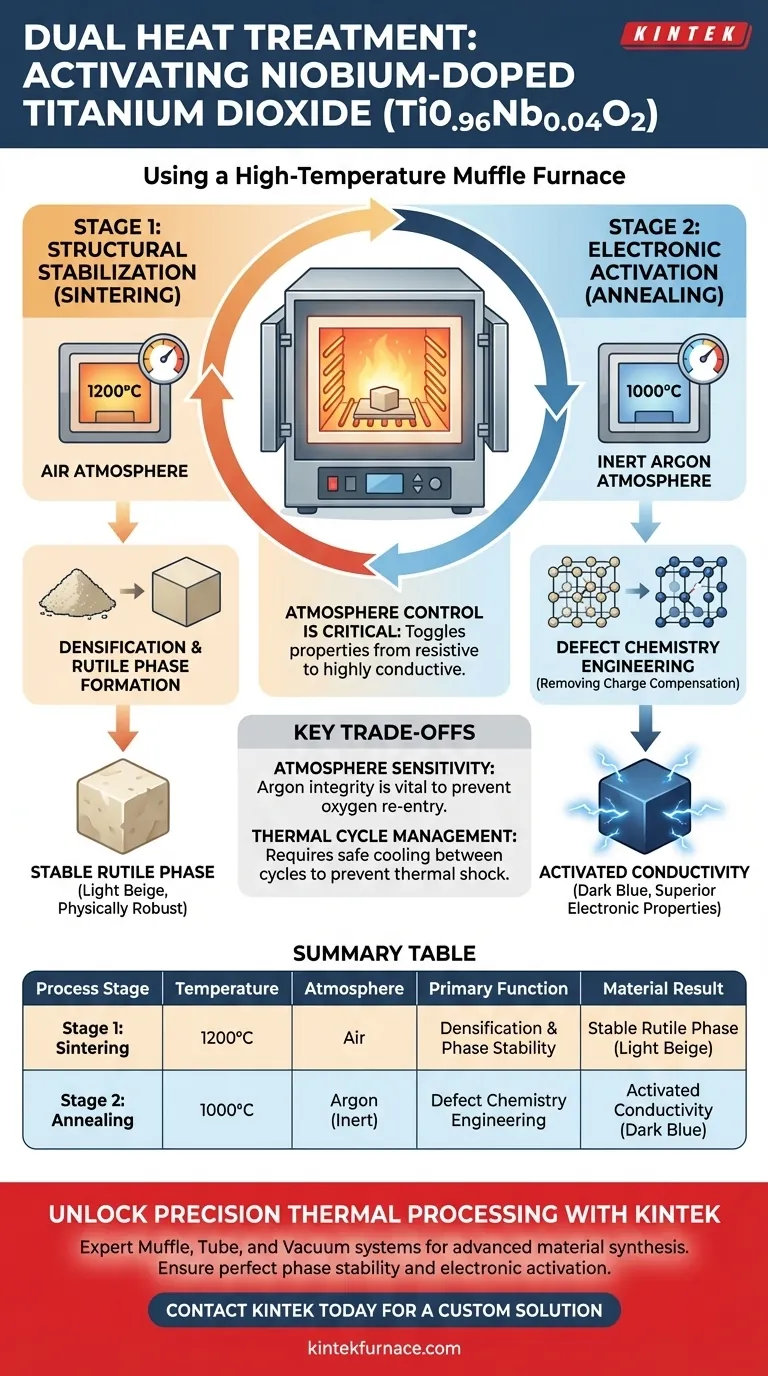
A high-temperature muffle furnace serves as the critical reaction chamber for transforming niobium-doped titanium dioxide (Ti0.96Nb0.04O2) from a standard oxide into a functional electronic material. It executes a two-step thermal protocol: first ensuring structural integrity through densification sintering at 1200°C in air, and subsequently activating electronic conductivity via secondary annealing at 1000°C in an inert argon atmosphere.
The muffle furnace’s ability to switch between oxidizing and inert atmospheres is the defining factor in this process. By controlling the environment, you effectively toggle the material's properties from a resistive state to a highly conductive state through precise defect engineering.

Stage 1: Structural Stabilization
Densification Sintering
The first function of the furnace is to establish the physical structure of the material. By heating the sample to 1200°C in a standard air atmosphere, the furnace promotes densification.
Formation of the Rutile Phase
This high-heat environment forces the material to sinter, locking it into a stable rutile phase. At this stage, the material is physically robust but has not yet achieved its desired electronic properties.
Radiant Heating Mechanism
Because a muffle furnace uses radiant heat from its walls rather than direct flame contact, the sample is protected from combustion contaminants. This ensures that the rutile phase formed is chemically pure.
Stage 2: Electronic Activation
Secondary Inert Annealing
The second, more specialized function of the furnace is to facilitate annealing at 1000°C under an argon atmosphere. This step is not about physical structure, but about chemical modification.
Adjusting Defect Chemistry
The inert argon environment is critical for adjusting the defect chemical state of the material. It works by removing charge compensation effects that are caused by titanium vacancies.
The Visual and Electrical Transformation
This chemical shift produces an immediate physical change: the material transitions from light beige to dark blue. This color change indicates that superior electronic conductivity has been successfully activated.
Understanding the Trade-offs
Atmosphere Sensitivity
The success of the second stage relies entirely on the integrity of the inert atmosphere. If the muffle furnace cannot maintain a pure argon environment, oxygen will re-enter the system, preventing the removal of titanium vacancies and failing to activate conductivity.
Thermal Cycle Management
Running two distinct high-temperature cycles (1200°C and 1000°C) places significant thermal stress on both the sample and the heating elements. Users must account for the time required to cool the furnace safely between the air sintering phase and the argon annealing phase to prevent thermal shock.
Optimizing Your Heat Treatment Strategy
To achieve the best results with niobium-doped titanium dioxide, align your furnace settings with your specific material goals:
- If your primary focus is structural integrity: Prioritize the initial 1200°C air sintering cycle to maximize density and ensure a stable rutile phase.
- If your primary focus is electronic conductivity: Ensure your furnace seal is impeccable during the 1000°C argon step to fully eliminate charge compensation effects.
Mastering the atmosphere within the furnace is just as critical as controlling the temperature.
Summary Table:
| Process Stage | Temperature | Atmosphere | Primary Function | Material Result |
|---|---|---|---|---|
| Stage 1: Sintering | 1200°C | Air | Densification & Phase Stability | Stable Rutile Phase (Light Beige) |
| Stage 2: Annealing | 1000°C | Argon (Inert) | Defect Chemistry Engineering | Activated Conductivity (Dark Blue) |
Unlock Precision Thermal Processing with KINTEK
Advanced material synthesis like niobium-doped titanium dioxide requires absolute control over atmosphere and thermal cycles. KINTEK provides industry-leading Muffle, Tube, and Vacuum systems designed to meet these rigorous demands.
Backed by expert R&D and manufacturing, our high-temperature furnaces are fully customizable to ensure your laboratory achieves perfect phase stability and electronic activation every time. Whether you need precise atmosphere switching or superior temperature uniformity, we deliver the tools for your success.
Ready to elevate your research? Contact KINTEK today for a custom furnace solution!
Visual Guide
References
- Tomoyuki Shiraiwa, Takahisa Omata. Enhanced Proton Transport in Nb-Doped Rutile TiO<sub>2</sub>: A Highly Useful Class of Proton-Conducting Mixed Ionic Electronic Conductors. DOI: 10.1021/jacs.5c05805
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What role does a high-temperature laboratory box furnace play in the sintering process of refractory bricks?
- Why is a high-temperature muffle furnace required for the roasting of activated fly ash? Unlock Efficient Phase Changes
- How do high-precision heating furnaces ensure quality during high-temperature capillary imprinting? | KINTEK
- How does the programmable temperature control of a high-precision box resistance furnace influence the properties of pyrolyzed composite materials?
- What is the function of a high-temperature box furnace in Cu-Ni-P alloy annealing? Optimize Your Cold Rolling Results
- What core roles does a muffle furnace play in the crystal growth of NaNbO3:Pr3+? Enhance Your Material Synthesis
- How is a laboratory muffle furnace used in 3D-printed PP-CF cross-linking? Achieve Thermal Stability at 150 °C
- How is a muffle furnace utilized during the raw material preparation stage? Optimize Your Lab Results Today



















