At the heart of the CVD process, vapor-phase precursors serve as the fundamental building blocks for creating thin films. These are gaseous chemical compounds that carry the specific atoms required for the final material. They are transported into a reaction chamber where, under controlled conditions, they react and deposit a solid layer onto a target surface, known as a substrate.
Precursors are far more than just delivery vehicles for atoms. Their specific chemical properties—volatility, reactivity, and purity—are the primary control levers that dictate the entire Chemical Vapor Deposition (CVD) process, from the quality of the final film to the efficiency and safety of the operation.

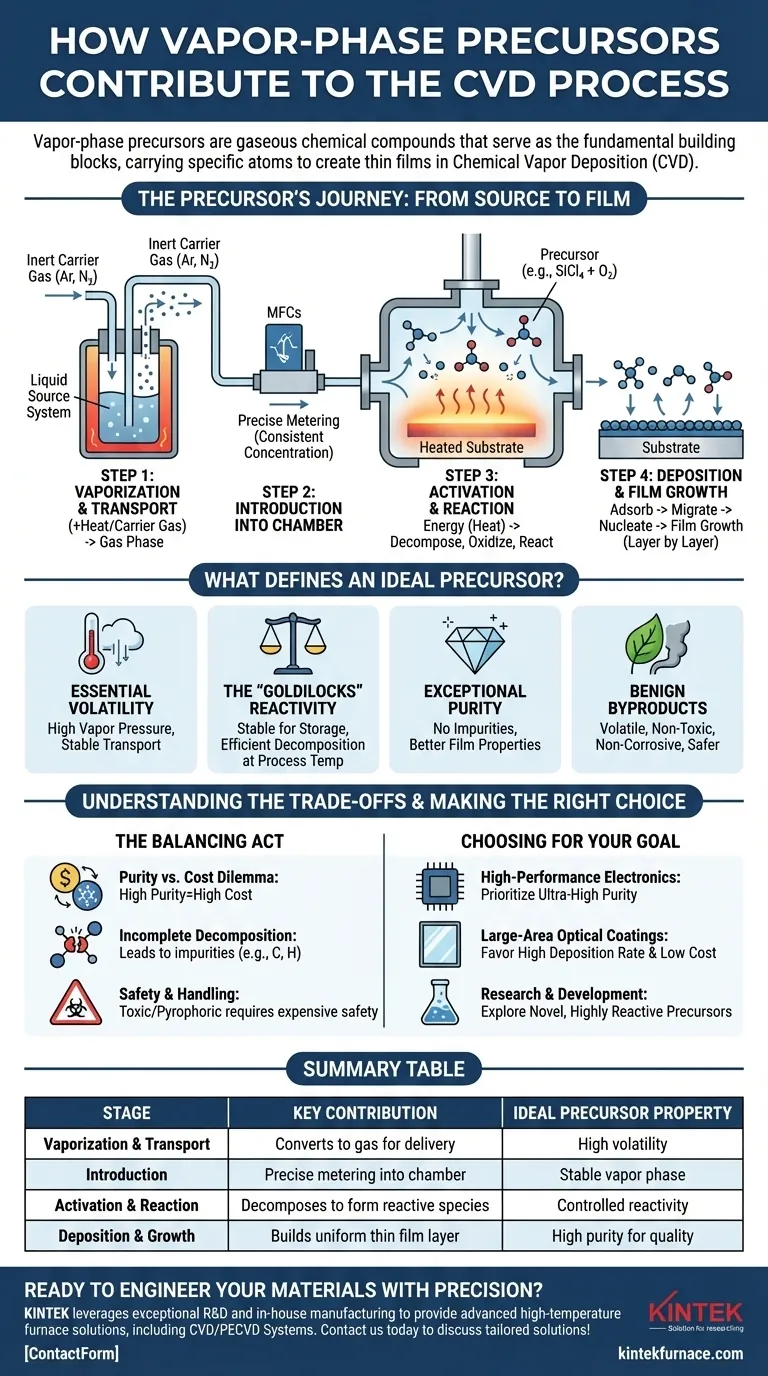
The Precursor's Journey: From Source to Film
The contribution of a precursor is best understood by following its path through the four critical stages of the CVD process.
Step 1: Vaporization and Transport
A precursor must be volatile, meaning it can be easily and stably converted into a gas. This is the "vapor-phase" in CVD.
For precursors that are liquid or solid at room temperature, they are often gently heated or have an inert carrier gas (like Argon or Nitrogen) bubbled through them to carry their vapor into the system's gas lines.
Step 2: Introduction into the Reaction Chamber
Once in a gaseous state, the precursor is precisely metered into the reaction chamber using components like mass flow controllers (MFCs).
This precise control is critical for maintaining a consistent concentration of reactants, which directly influences the deposition rate and uniformity of the resulting film.
Step 3: Activation and Reaction
Inside the chamber, energy—typically from a heated substrate—activates the precursor molecules. This energy breaks their chemical bonds, causing them to decompose, oxidize, or react with other introduced gases.
For example, to deposit silicon dioxide (SiO₂), the precursor silicon tetrachloride (SiCl₄), which carries the silicon atoms, is introduced with oxygen (O₂). The heat causes them to react, forming the desired SiO₂ molecules.
Step 4: Deposition and Film Growth
The newly formed solid molecules or reactive species adsorb (stick) onto the hot substrate surface. They migrate across the surface, find energetically favorable sites, and bond together.
This process, called nucleation and growth, builds up layer by layer to form a continuous, solid thin film with a controlled thickness and structure.
What Defines an Ideal Precursor?
The choice of precursor is one of the most critical decisions in designing a CVD process. The ideal chemical exhibits a specific set of characteristics.
Essential Volatility
The precursor must have a high enough vapor pressure to be transported into the reactor as a gas at a reasonable rate without decomposing prematurely in the gas lines.
The "Goldilocks" Reactivity
A precursor must be stable enough for storage and transport but reactive enough to decompose efficiently at the desired process temperature. If it's too stable, the process requires impractically high temperatures. If it's too reactive, it can decompose in the gas phase before reaching the substrate, forming powders instead of a quality film.
Exceptional Purity
Any impurity within the precursor chemical (e.g., unwanted metals or organic compounds) will likely be incorporated into the final film. These impurities can severely degrade the film's electrical, optical, or mechanical properties.
Benign Byproducts
The chemical reactions that break down the precursor also create byproducts. In the deposition of SiO₂ from SiCl₄, for example, corrosive chlorine gas (Cl₂) is a byproduct. Ideal precursors generate byproducts that are volatile, non-toxic, and non-corrosive, simplifying removal and improving process safety.
Understanding the Trade-offs
Choosing a precursor is rarely a simple task and almost always involves balancing competing factors.
The Purity vs. Cost Dilemma
Ultra-high purity precursors are essential for demanding applications like microelectronics, but they are significantly more expensive. For less critical applications, a lower-purity, more cost-effective precursor may be sufficient.
Incomplete Decomposition
If process conditions (like temperature or pressure) are not perfectly optimized for the chosen precursor, it may not break down completely. This can lead to the unintentional incorporation of elements like carbon or hydrogen into the film, which is a common challenge with metal-organic precursors (MOCVD).
Safety and Handling
Many of the most effective precursors are highly toxic, pyrophoric (ignite spontaneously in air), or corrosive. Using them requires extensive and costly safety infrastructure, including sealed gas cabinets, hazardous gas detectors, and abatement systems to treat the exhaust.
Making the Right Choice for Your Goal
Your application's primary objective will guide your precursor selection strategy.
- If your primary focus is high-performance electronics: You must prioritize ultra-high purity precursors to achieve the required electrical properties and minimize device-killing defects.
- If your primary focus is large-area optical coatings: You will likely favor precursors that offer high deposition rates and lower material costs, even if it requires managing more challenging byproducts.
- If your primary focus is research and development: You might explore novel, highly reactive precursors to enable film growth at lower temperatures, which allows deposition on sensitive substrates like plastics.
By understanding the precursor's role and its inherent properties, you move from simply running a process to intentionally engineering the materials of the future.
Summary Table:
| Stage | Key Contribution | Ideal Precursor Property |
|---|---|---|
| Vaporization & Transport | Converts to gas for delivery | High volatility |
| Introduction | Precise metering into chamber | Stable vapor phase |
| Activation & Reaction | Decomposes to form reactive species | Controlled reactivity |
| Deposition & Growth | Builds uniform thin film layer | High purity for quality |
Ready to engineer your materials with precision? KINTEK leverages exceptional R&D and in-house manufacturing to provide advanced high-temperature furnace solutions, including CVD/PECVD Systems. Our Muffle, Tube, Rotary, Vacuum & Atmosphere Furnaces, combined with deep customization capabilities, ensure your unique experimental requirements are met. Contact us today to discuss how our tailored solutions can enhance your CVD process efficiency and safety!
Visual Guide
Related Products
- RF PECVD System Radio Frequency Plasma Enhanced Chemical Vapor Deposition
- Inclined Rotary Plasma Enhanced Chemical Deposition PECVD Tube Furnace Machine
- Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
- Inclined Rotary Plasma Enhanced Chemical Deposition PECVD Tube Furnace Machine
- Custom Made Versatile CVD Tube Furnace Chemical Vapor Deposition CVD Equipment Machine
People Also Ask
- What are the main components of a PECVD system? Unlock Low-Temperature Thin Film Deposition
- What is the second benefit of deposition within a discharge in PECVD? Enhance Film Quality with Ion Bombardment
- How does plasma enhanced CVD work? Achieve Low-Temperature, High-Quality Thin Film Deposition
- What role does PECVD play in optical coatings? Essential for Low-Temp, High-Precision Film Deposition
- What gases are used in the PECVD system? Optimize Thin Film Deposition with Precise Gas Selection



















