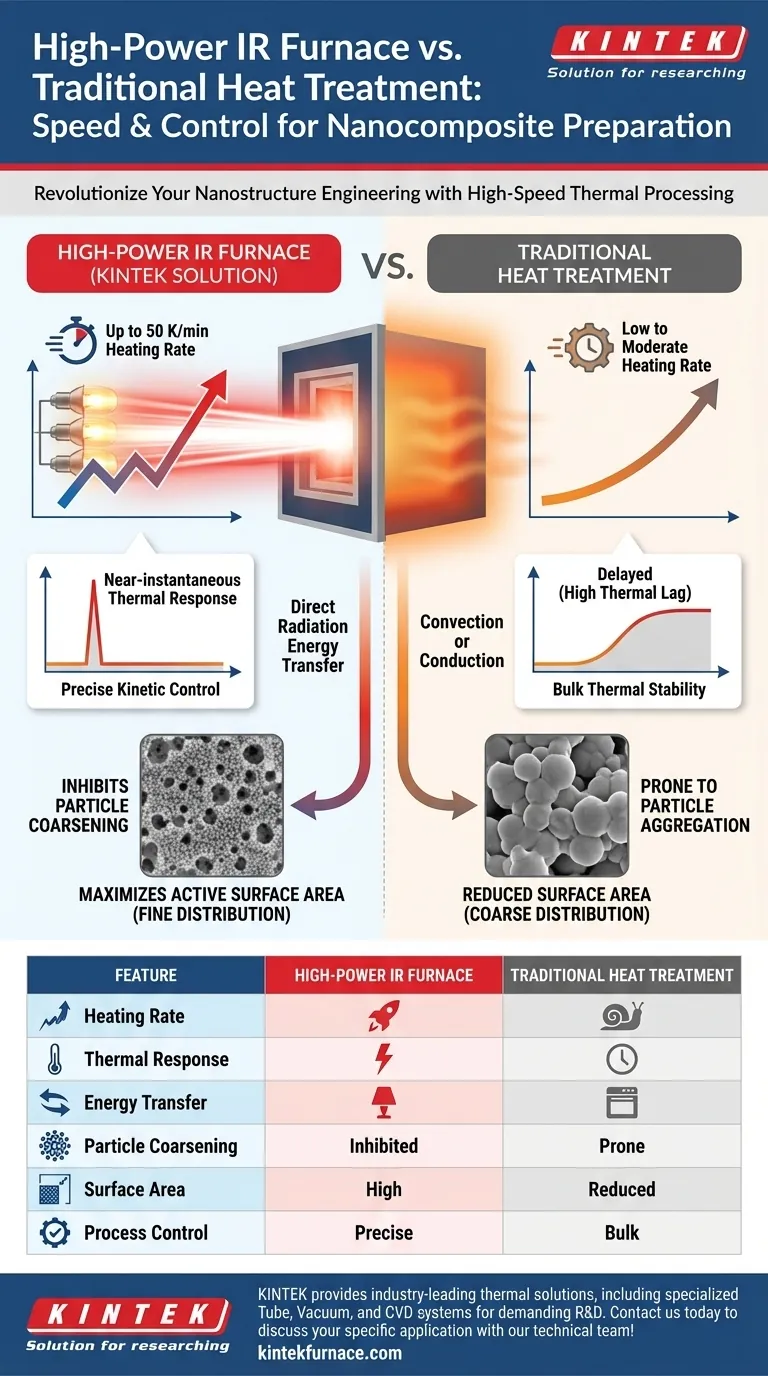
High-power infrared (IR) furnaces fundamentally outperform traditional heat treatment equipment regarding heating speed and kinetic control. Utilizing high-power halogen lamps, these systems achieve exceptional heating rates of up to 50 K/min with near-instantaneous response times, contrasting sharply with the slower thermal ramp-up typical of conventional furnaces.
Core Insight: The decisive advantage of IR heating lies in its ability to alter pyrolysis kinetics. By bypassing the slow heating phases where particles tend to aggregate, IR furnaces effectively inhibit metal particle coarsening, producing nanocomposites with finer particle distributions and significantly higher active surface areas.
The Mechanics of High-Speed Thermal Processing
To understand the superiority of IR furnaces for nanocomposite preparation, one must look at the heating mechanism itself. Traditional equipment often relies on convection or conduction, which introduces thermal lag.
Achieving Rapid Heating Rates
IR furnaces utilize high-power halogen lamps to transfer energy via radiation. This allows the system to achieve heating rates as high as 50 K/min.
This rapid influx of thermal energy minimizes the time a sample spends in intermediate temperature zones. In traditional processing, these intermediate zones are often where unwanted structural changes begin to occur.
Instantaneous Thermal Response
A distinct advantage of this technology is its response time. Because the heat source is light-based, the thermal response is near-instantaneous.
This allows for precise manipulation of the temperature profile. Operators can start and stop heating cycles with immediate effect, providing a level of process control that massive, thermally sluggish resistive furnaces cannot match.
Impact on Material Microstructure
The physical properties of porous nanocomposites, such as those derived from ZIF-67, are dictated by how they are heated. The method of heating is not just about reaching a temperature; it is about how the material behaves on the way to that temperature.
Controlling Pyrolysis Kinetics
The rapid heating capability of IR furnaces provides superior control over pyrolysis kinetics.
When processing precursors like ZIF-67, the rate at which the organic framework breaks down determines the final metal structure. Fast heating rates lock in desirable kinetic pathways that slow heating rates might miss.
Inhibiting Particle Coarsening
One of the primary failure modes in traditional heat treatment is "coarsening." This occurs when metal particles aggregate and grow larger during prolonged exposure to high temperatures.
High-power IR heating effectively inhibits excessive coarsening. By minimizing the time window in which particles can migrate and merge, the process preserves the nanostructure.
Maximizing Active Surface Area
The direct result of preventing coarsening is a finer particle distribution.
Smaller, discrete particles translate directly to a higher active surface area. For catalytic or storage applications, this surface area is the critical metric defining the material's performance.
Understanding the Trade-offs
While IR heating offers distinct advantages for nanostructure preservation, it represents a specific tool for a specific set of challenges.
Line-of-Sight Limitations
IR heating is radiative, meaning it relies on line-of-sight transfer. Unlike a convection oven which surrounds a part with hot air, IR energy must reach the surface directly. Complex geometries may require careful sample positioning to ensure uniform exposure.
Sensitivity to Process Variables
The "instantaneous response" of IR systems is a double-edged sword. While it offers control, it lacks the thermal buffer of a massive brick furnace. Fluctuations in power or control loops manifest immediately in the sample temperature, requiring robust control systems.
Making the Right Choice for Your Goal
When selecting between an IR furnace and traditional heat treatment for nanocomposite preparation, consider your specific material requirements.
- If your primary focus is maximizing active surface area: Choose the IR furnace to utilize rapid heating rates (50 K/min) that prevent particle agglomeration and coarsening.
- If your primary focus is precise kinetic control: Rely on the near-instantaneous response of halogen lamps to strictly dictate the pyrolysis profile of precursors like ZIF-67.
By leveraging the speed of high-power IR sources, you transition from simply heating a material to engineering its nanostructure through kinetic control.
Summary Table:
| Feature | High-Power IR Furnace | Traditional Heat Treatment |
|---|---|---|
| Heating Rate | Up to 50 K/min (Rapid) | Low to Moderate (Slow) |
| Thermal Response | Near-instantaneous | Delayed (High Thermal Lag) |
| Energy Transfer | Radiation (Halogen Lamps) | Convection or Conduction |
| Particle Coarsening | Effectively Inhibited | Highly Prone to Aggregation |
| Surface Area | Maximum Active Surface Area | Reduced due to Coarsening |
| Process Control | High Kinetic Precision | Bulk Thermal Stability |
Revolutionize Your Nanostructure Engineering with KINTEK
Don't let traditional heating limitations compromise your material performance. KINTEK provides industry-leading thermal solutions, including specialized Tube, Vacuum, and CVD systems designed for the most demanding R&D requirements.
Whether you need to inhibit particle coarsening or achieve precise pyrolysis kinetics, our expert manufacturing and customizable high-temperature furnaces are engineered to meet your unique laboratory needs.
Ready to scale your research with superior precision? Contact us today to discuss your specific application with our technical team!
Visual Guide
References
- D. G. Muratov, А. В. Зорин. Metal-organic frameworks and composites on their basis: structure, synthesis methods, electrochemical properties and application prospects (a review). DOI: 10.3897/j.moem.10.2.126396
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- How does Plasma Flash Sintering (PFS) equipment enable the stabilization of metastable phases? Defy Thermal Limits
- Why are User-Defined Functions (UDFs) necessary for modeling complex combustion? Unlock Precision in Furnace Simulation
- How does a constant temperature and humidity curing chamber contribute to alkali-activated material performance?
- What conditions does an autoclave provide for MoS2 hydrothermal synthesis? Achieve Optimal MoS2 Nanosheet Growth
- Why is an 800 °C heat treatment for Ti6Al4V additive manufacturing necessary? Unlock Ductility & Relieve Stress
- Why is preheating a metal mold to 660 °C necessary for Al/Cu bimetallic composites? Unlock Strong Chemical Bonding
- Why are high frequencies used in induction heating? For Precise, Rapid Surface Heating
- Why is a planetary ball mill required for processing activated carbon? Achieve <30μm Particles for Superior Slurry



















