When a chemically active metal is heated in an air furnace, it initiates a series of detrimental chemical reactions with the surrounding atmosphere. These reactions occur both on the surface, forming an oxide film or scale, and within the metal's internal structure as gases like oxygen, nitrogen, and hydrogen are absorbed. The result is a significant deterioration of the metal's original mechanical properties and surface finish.
Heating a reactive metal in open air is not a passive process. The furnace atmosphere acts as an aggressive chemical agent, fundamentally altering the metal by creating brittle surface layers and introducing internal impurities that compromise its structural integrity.

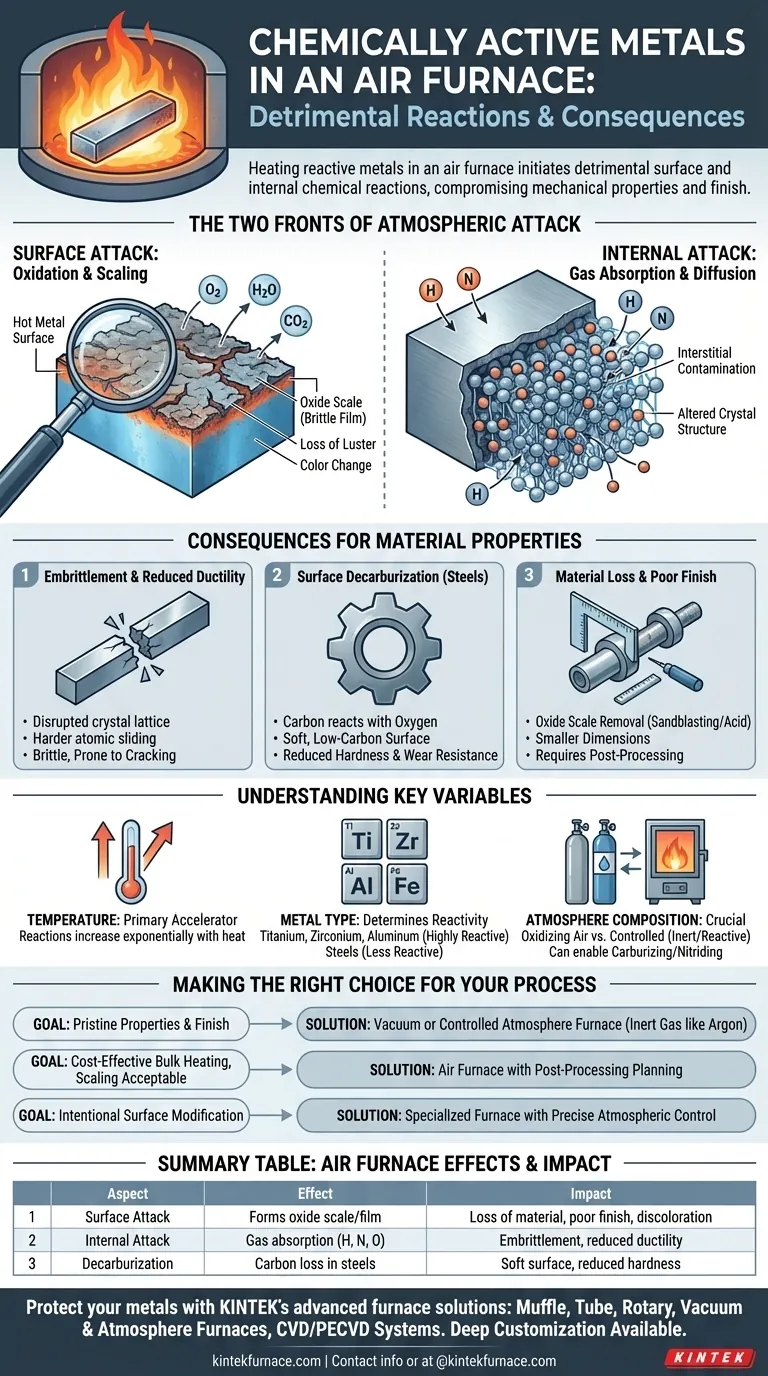
The Two Fronts of Atmospheric Attack
When a metal is heated, its atoms vibrate more intensely, making it far more susceptible to chemical reactions. An air furnace provides a ready supply of reactive gases that attack the metal in two distinct ways.
Surface Attack: Oxidation and Scaling
The most visible effect is oxidation. The hot metal surface reacts readily with oxygen, water vapor, and carbon dioxide present in the air.
This reaction forms a brittle, often flaky, layer of metal oxide known as scale or an oxide film. This process is responsible for the metal losing its metallic luster and changing color.
Internal Attack: Gas Absorption and Diffusion
Simultaneously, smaller atomic elements like hydrogen (from water vapor) and nitrogen can be absorbed by the surface. At high temperatures, these atoms diffuse from the surface deep into the metal's interior crystalline structure.
This internal contamination is often invisible but is particularly damaging, as it alters the metal's properties from within.
The Consequences for Material Properties
These chemical changes are not merely cosmetic. They have direct, negative consequences on the performance and reliability of the metallic component.
Embrittlement and Reduced Ductility
The absorption of gases, particularly hydrogen and nitrogen, disrupts the metal's crystal lattice. This is often called interstitial contamination.
This disruption makes it harder for the atomic layers to slide past one another, causing the metal to become significantly more brittle and lose its ductility. A brittle metal is more likely to crack or fracture under stress instead of bending.
Surface Decarburization (in Steels)
For carbon steels, the oxygen in the furnace atmosphere can react with the carbon near the surface of the part. This reaction "burns off" the carbon, leaving a soft, low-carbon iron layer.
This decarburization is highly undesirable in applications requiring a hard, wear-resistant surface, such as gears or bearings.
Material Loss and Poor Finish
The oxide scale that forms on the surface represents a loss of the base metal. This scale is typically removed after heat treatment through processes like sandblasting or acid pickling, resulting in a final part that is smaller than its original dimensions.
Understanding the Key Variables
The severity of these effects is not constant; it depends on a few critical factors that you can often control.
Temperature is the Primary Accelerator
The rate of all these chemical reactions—oxidation, diffusion, and decarburization—increases exponentially with temperature. A small increase in furnace temperature can cause a dramatic increase in atmospheric attack.
Metal Type Determines Reactivity
Metals like titanium, zirconium, and aluminum are extremely reactive and are highly susceptible to gas absorption and oxidation. Steels are also reactive, but generally less so than this group. The choice of heating method must account for the metal's inherent reactivity.
Atmosphere Composition is Crucial
While an air furnace is inherently oxidizing, slight variations in the atmosphere can change the outcome. An atmosphere with excess carbon monoxide (CO) or methane (CH4) can actually add carbon to the surface of steel (carburizing), which is the opposite of decarburization. This highlights that the furnace atmosphere is an active chemical variable.
Making the Right Choice for Your Process
Understanding these reactions is key to selecting the appropriate heating method for your goal.
- If your primary focus is preserving pristine material properties and surface finish: An air furnace is unsuitable. You must use a vacuum furnace or a controlled furnace with an inert gas atmosphere (like argon) to protect the metal.
- If your primary focus is cost-effective bulk heating and some surface scaling is acceptable: An air furnace can be used, but you must plan for post-processing steps like machining or cleaning to remove the damaged surface layer.
- If your goal is to intentionally modify the surface (e.g., case hardening): You must use a specialized furnace with precise atmospheric controls to introduce specific elements like carbon (carburizing) or nitrogen (nitriding) in a predictable way.
Ultimately, controlling the furnace atmosphere is not an afterthought; it is a critical parameter for achieving the desired metallurgical outcome.
Summary Table:
| Aspect | Effect in Air Furnace | Impact on Metal |
|---|---|---|
| Surface Attack | Forms oxide scale/film | Loss of material, poor finish, discoloration |
| Internal Attack | Gas absorption (H, N, O) | Embrittlement, reduced ductility |
| Decarburization | Carbon loss in steels | Soft surface, reduced hardness |
| Key Variables | Temperature, metal type, atmosphere | Determines severity of damage |
Protect your metals from atmospheric damage with KINTEK's advanced furnace solutions. Leveraging exceptional R&D and in-house manufacturing, we offer Muffle, Tube, Rotary, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems, with strong deep customization to meet your unique experimental needs. Contact us today to enhance your lab's efficiency and material quality!
Visual Guide
Related Products
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
People Also Ask
- What does inert mean in furnace atmospheres? Protect materials from oxidation with inert gases.
- What is the use of nitrogen in furnace? Prevent Oxidation for Superior Heat Treatment
- What are the environmental benefits of using inert gases in furnaces? Reduce Waste and Emissions for a Greener Process
- How does a chemically inert atmosphere function in a furnace? Prevent Oxidation and Ensure Material Purity
- How does an inert atmosphere prevent oxidation? Shield Materials from Oxygen Damage



















