In chemical vapor deposition (CVD) for graphene, quartz is the standard material for reaction chambers primarily due to two properties: its extreme thermal stability and its chemical inertness. At the high temperatures required for synthesis (often exceeding 1000°C), quartz remains structurally sound and does not react with the volatile precursor gases, ensuring the purity of the final graphene film.
The core challenge in graphene CVD is not just growing the material, but doing so with near-perfect atomic purity. Quartz is chosen because it acts as a chemically invisible and structurally stable container, ensuring the reaction between the gas and the metal catalyst occurs precisely as intended, without interference from the chamber itself.

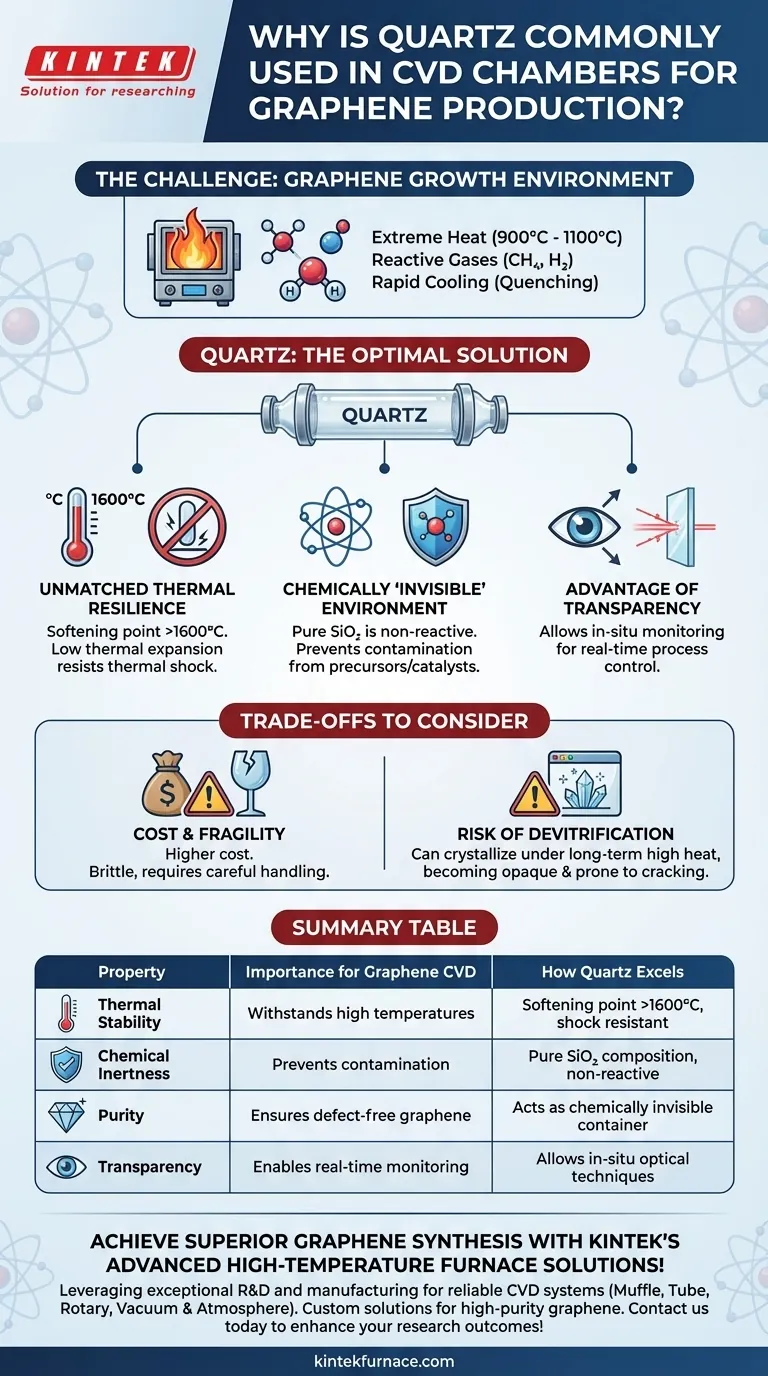
The Core Requirements of a Graphene CVD Chamber
To understand why quartz is the default choice, we must first define the harsh requirements of the graphene growth environment. The chamber is not merely a container; it is a critical component of the reaction system.
Withstanding Extreme Heat
Graphene CVD is a high-temperature process, typically running between 900°C and 1100°C. The chamber material must maintain its structural integrity without softening, deforming, or failing.
This high-temperature requirement immediately disqualifies most common lab materials, including borosilicate glass (like Pyrex), which softens around 820°C.
Maintaining Chemical Purity
The process involves highly reactive gases, such as methane (CH₄) and hydrogen (H₂). The chamber material must be chemically inert, meaning it will not react with these gases or the copper or nickel catalyst foil.
Any reaction would introduce contaminants into the graphene lattice, creating defects and ruining its electronic properties. The chamber must act as a perfectly clean stage for the chemical reaction.
Surviving Thermal Shock
At the end of the growth cycle, the system is often cooled rapidly in a process called quenching. This rapid temperature change induces immense stress on the material.
A suitable chamber must have a very low coefficient of thermal expansion to resist cracking or shattering under this thermal shock.
Why Quartz Excels in These Areas
Quartz (specifically fused quartz or fused silica) possesses a unique combination of properties that makes it almost perfectly suited for the demands of graphene CVD.
Unmatched Thermal Resilience
Quartz has an extremely high softening point of over 1600°C, providing a massive safety and operational margin for processes running at 1000°C.
Furthermore, its exceptionally low coefficient of thermal expansion makes it highly resistant to thermal shock. It can withstand the rapid heating and cooling cycles of CVD without fracturing, ensuring reliability and safety.
A Chemically 'Invisible' Environment
Fused quartz is composed of very pure silicon dioxide (SiO₂), which is exceptionally non-reactive. It does not react with hydrocarbon precursors, hydrogen, or the metal catalysts used in graphene growth.
This inertness is the single most important factor for ensuring the growth of high-purity graphene. It prevents the chamber walls from becoming an unintentional source of contamination.
The Advantage of Transparency
As a bonus, the optical transparency of quartz allows researchers to use in-situ monitoring techniques. Lasers and spectrometers can be used to observe the growth process in real-time through the chamber walls, enabling better process control and optimization.
Understanding the Trade-offs
While quartz is the ideal material, it is not without its limitations. Understanding these trade-offs is crucial for practical lab work and process design.
The Primary Limitation: Cost
High-purity fused quartz is significantly more expensive than other types of glassware, such as borosilicate. This cost can be a factor for large-scale production or budget-constrained research environments.
Mechanical Fragility
Like any glass, quartz is brittle and must be handled with care. It is susceptible to breaking from mechanical shock, and any surface scratches can become stress points that lead to failure under thermal cycling.
Risk of Devitrification
Over very long periods at high temperatures, particularly in the presence of certain surface contaminants (alkali metals), quartz can begin to crystallize in a process called devitrification. This crystallized form is opaque and has a higher thermal expansion, making it much more prone to cracking.
Making the Right Choice for Your Goal
Selecting the right chamber material is about controlling variables to achieve a specific outcome.
- If your primary focus is producing the highest-purity, defect-free graphene for research or electronics: Fused quartz is non-negotiable due to its superior inertness and thermal stability.
- If your primary focus is education, rapid prototyping, or lower-temperature processes: Understanding why quartz is ideal helps you recognize the compromises made when using less-optimal materials.
Ultimately, the choice of quartz is a strategic decision to eliminate the reaction chamber as a variable, enabling reproducible and high-quality graphene synthesis.
Summary Table:
| Property | Importance for Graphene CVD | How Quartz Excels |
|---|---|---|
| Thermal Stability | Withstands high temperatures (900-1100°C) without deformation | Softening point >1600°C, low thermal expansion for shock resistance |
| Chemical Inertness | Prevents contamination from reactive gases and catalysts | Pure SiO₂ composition, non-reactive with precursors and catalysts |
| Purity | Ensures defect-free graphene with optimal electronic properties | Acts as a chemically invisible container, no unwanted reactions |
| Transparency | Enables real-time monitoring of growth process | Allows in-situ optical techniques for better control |
Achieve superior graphene synthesis with KINTEK's advanced high-temperature furnace solutions! Leveraging exceptional R&D and in-house manufacturing, we provide diverse laboratories with reliable CVD systems, including Muffle, Tube, Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems. Our strong deep customization capability ensures precise alignment with your unique experimental needs, such as high-purity graphene production. Contact us today to discuss how our tailored solutions can enhance your research outcomes and efficiency!
Visual Guide
Related Products
- Custom Made Versatile CVD Tube Furnace Chemical Vapor Deposition CVD Equipment Machine
- Multi Heating Zones CVD Tube Furnace Machine for Chemical Vapor Deposition Equipment
- Vertical Laboratory Quartz Tube Furnace Tubular Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- Inclined Rotary Plasma Enhanced Chemical Deposition PECVD Tube Furnace Machine
People Also Ask
- Which industries and research fields benefit from CVD tube furnace sintering systems for 2D materials? Unlock Next-Gen Tech Innovations
- What role do CVD tube furnace sintering systems play in 2D material synthesis? Enabling High-Quality Atomic Layer Growth
- Why are CVD tube furnace sintering systems indispensable for 2D material research and production? Unlock Atomic-Scale Precision
- Why are advanced materials and composites important? Unlock Next-Gen Performance in Aerospace, Auto, and More
- Why is the tube design important in CVD furnaces? Ensure Uniform Deposition for High-Quality Films



















