In metallurgy, control is everything. Vacuum melting is essential for certain metal alloys because it removes air and other atmospheric impurities from the environment. This prevents unwanted chemical reactions, primarily oxidation, which can compromise an alloy's integrity, alter its chemical composition, and introduce critical defects into the final material.
Moving beyond simply preventing contamination, operating in a vacuum gives metallurgists precise control over the entire melting process. This control is the key to achieving superior purity, compositional accuracy, and ultimately, higher-performance materials demanded by advanced industries.

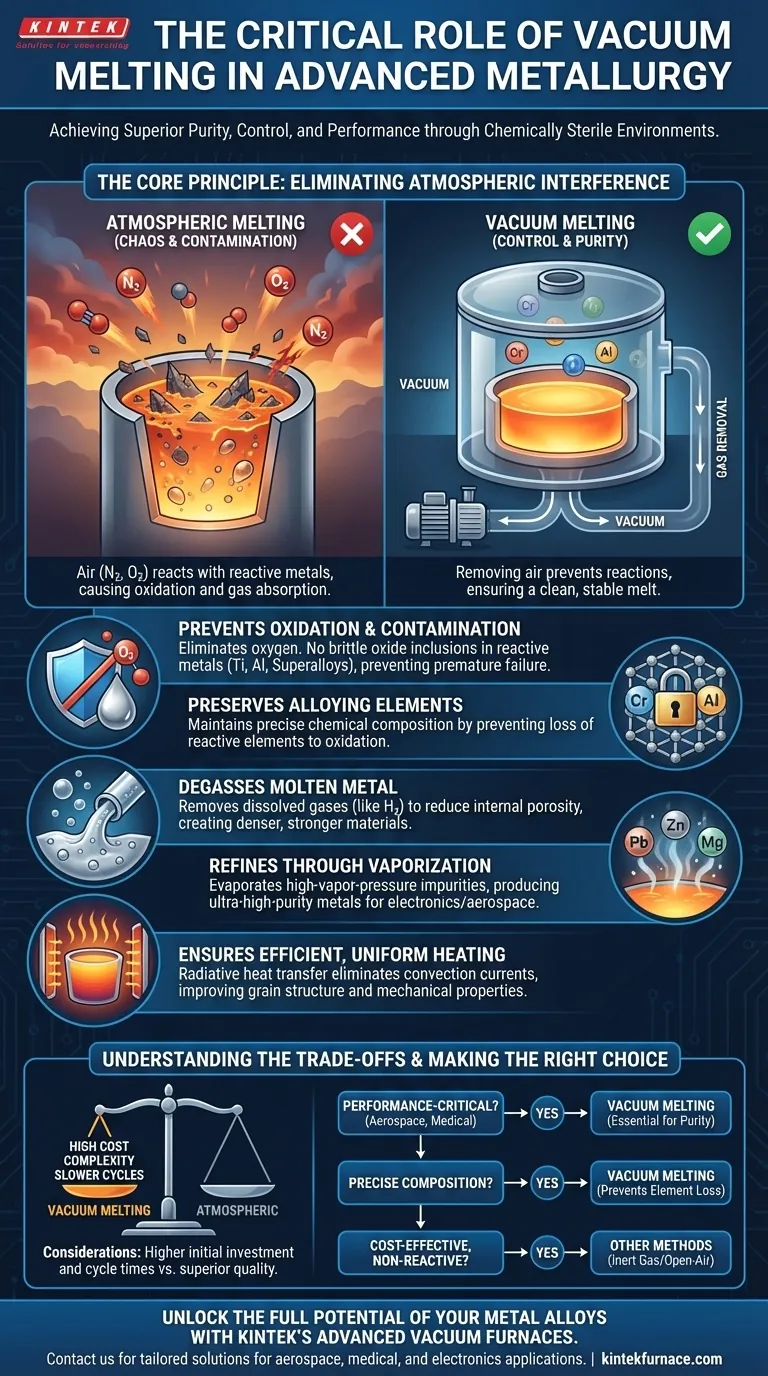
The Core Principle: Eliminating Atmospheric Interference
The fundamental reason for using a vacuum is to create a chemically sterile environment. Air, which is primarily nitrogen and oxygen, is highly reactive at the extreme temperatures required for melting metals.
Preventing Oxidation and Contamination
When reactive metals like titanium, aluminum, or nickel-based superalloys are melted in the presence of air, they readily form oxides. These oxides are brittle, ceramic-like inclusions that create weak points within the metal structure, leading to premature failure.
A vacuum environment effectively eliminates the oxygen available for these reactions, ensuring the molten metal remains clean and free from harmful oxide films and inclusions.
Preserving Alloying Elements
Many alloys rely on precise amounts of highly reactive elements (like chromium or aluminum) to achieve their desired properties. In an open-air melt, these elements can be preferentially lost to oxidation.
By removing the atmosphere, vacuum melting ensures these critical alloying elements remain in the melt at their intended concentrations, guaranteeing the final product has the correct chemical composition and performs as designed.
Enhancing Purity and Material Quality
Beyond preventing reactions with air, a vacuum actively refines the molten metal, improving its overall quality and consistency in several ways.
Degassing the Molten Metal
Liquid metals can dissolve significant amounts of gases, especially hydrogen. As the metal cools and solidifies, the solubility of these gases drops, causing them to be rejected from the metal and form internal porosity.
The low-pressure environment of a vacuum furnace effectively pulls these dissolved gases out of the molten bath, resulting in a denser, stronger final product free from gas-related defects.
Refining Through Vaporization
A vacuum lowers the boiling point of all elements. This principle is used to purify the alloy by encouraging impurity elements with high vapor pressure—such as lead, zinc, bismuth, and magnesium—to evaporate out of the melt.
This process, known as vacuum refining, is a powerful tool for producing ultra-high-purity metals required for demanding applications in electronics and aerospace.
Ensuring Efficient and Uniform Heating
In a vacuum, there is no air to transfer heat via convection. Heat is transferred almost entirely through radiation, which is a more direct and uniform method.
This leads to a more efficient melting process with fewer hot or cold spots in the crucible. This thermal consistency can improve the final grain structure and mechanical properties of the alloy.
Understanding the Trade-offs
While powerful, vacuum melting is not a universal solution. The decision to use it involves clear trade-offs between quality, cost, and complexity.
High Initial Cost and Complexity
Vacuum induction melting (VIM) furnaces are significantly more expensive to build and operate than their atmospheric counterparts. They require complex vacuum systems, instrumentation, and highly skilled operators to manage the process safely and effectively.
Slower Production Cycles
The process of pumping down the chamber to the required vacuum level adds considerable time to each melt cycle. For high-volume production of less demanding materials, this can create a significant bottleneck.
Not Necessary for All Alloys
For many common, non-reactive alloys like simple carbon steels or cast irons, the added expense and complexity of vacuum melting are unnecessary. Simpler methods using inert gas blankets (like argon) or even open-air melting can produce perfectly acceptable results for their intended applications.
Making the Right Choice for Your Goal
Selecting the correct melting environment depends entirely on the material being processed and the performance requirements of the final component.
- If your primary focus is performance-critical applications (aerospace, medical): You must use vacuum melting for reactive alloys like titanium or superalloys to guarantee the highest purity and prevent catastrophic material failures.
- If your primary focus is compositional accuracy: Vacuum melting is the best choice to prevent the loss of expensive or reactive alloying elements, ensuring the final chemistry is exactly as specified.
- If your primary focus is cost-effective production of non-reactive metals: An open-air or controlled-atmosphere furnace is likely the more practical and economical choice.
Ultimately, choosing to melt in a vacuum is a strategic decision to exert maximum control over a material's fundamental chemistry and final properties.
Summary Table:
| Benefit | Description |
|---|---|
| Prevents Oxidation | Eliminates oxygen to avoid brittle oxide inclusions in reactive metals like titanium and superalloys. |
| Preserves Alloying Elements | Maintains precise chemical composition by preventing loss of reactive elements like chromium or aluminum. |
| Degasses Molten Metal | Removes dissolved gases such as hydrogen to reduce porosity and increase material density and strength. |
| Refines Through Vaporization | Evaporates impurities like lead and zinc for ultra-high-purity metals in demanding industries. |
| Ensures Uniform Heating | Uses radiation for efficient, consistent melting, improving grain structure and mechanical properties. |
Unlock the Full Potential of Your Metal Alloys with KINTEK's Advanced Vacuum Furnaces
Are you working with reactive metals like titanium or superalloys and need to achieve superior purity, precise composition, and defect-free materials? KINTEK leverages exceptional R&D and in-house manufacturing to provide diverse laboratories with advanced high-temperature furnace solutions. Our product line, including Muffle, Tube, Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems, is complemented by strong deep customization capabilities to precisely meet your unique experimental requirements. Whether you're in aerospace, medical, or electronics, we can help you enhance material quality and performance. Contact us today to discuss how our tailored vacuum melting solutions can benefit your specific applications!
Visual Guide
Related Products
- Vacuum Induction Melting Furnace
- 600T Vacuum Induction Hot Press Vacuum Heat Treat and Sintering Furnace
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
People Also Ask
- How does vacuum melting technology contribute to sustainability? Boost Durability and Recycling Efficiency
- What is the purpose of vacuum melting, casting and re-melting equipment? Achieve High-Purity Metals for Critical Applications
- What are the core functions of the High Vacuum Induction Melting (VIM) furnace? Optimize DD5 Superalloy Purification
- What are some common applications of vacuum induction melting and casting (VIM&C)? Essential for Aerospace, Medical, and Nuclear Industries
- Why is a Vacuum Induction Melting (VIM) furnace essential? Unlock Purity for Aerospace and Semiconductors



















