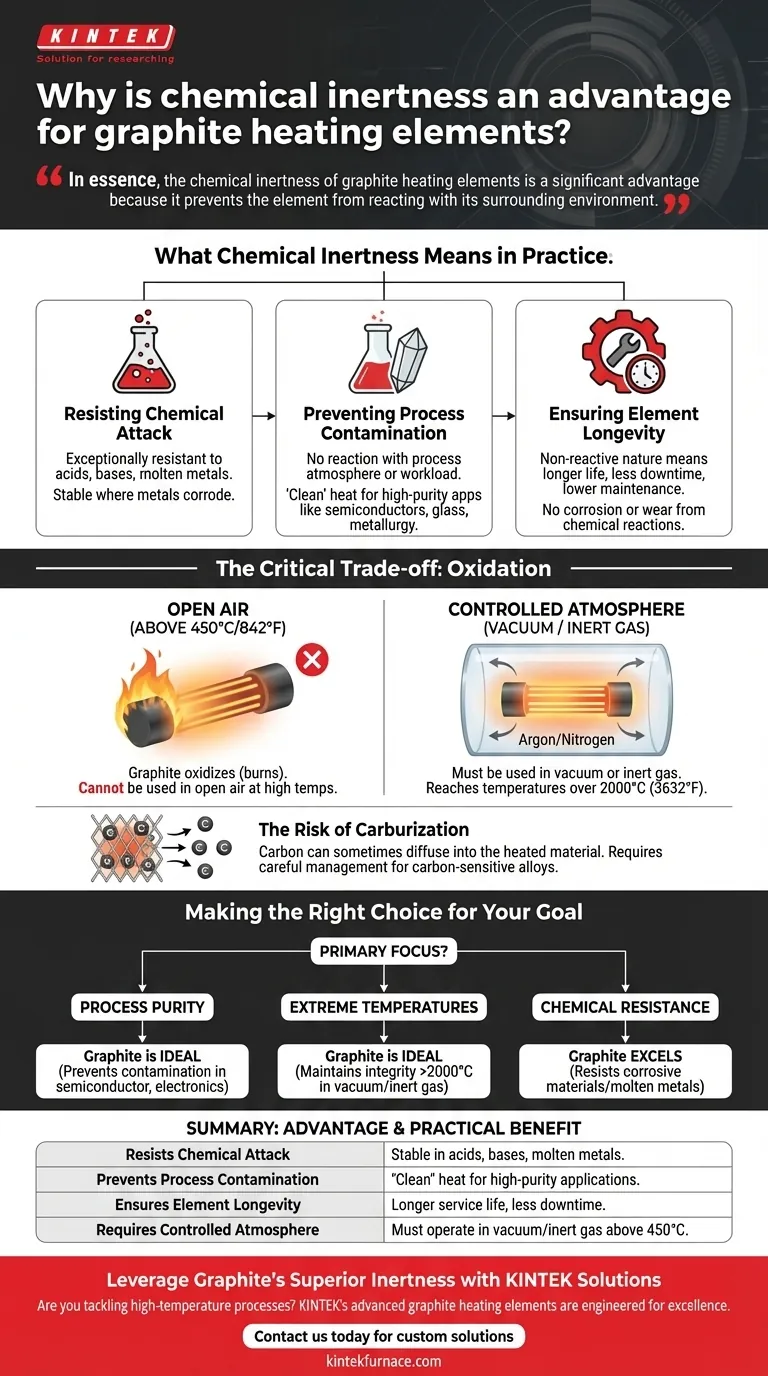
In essence, the chemical inertness of graphite heating elements is a significant advantage because it prevents the element from reacting with its surrounding environment. This non-reactivity ensures the purity of the material being heated while also protecting the element from corrosion, which dramatically increases its operational lifespan and reliability in high-temperature applications.
In the demanding world of high-temperature industrial processes, chemical reactions are the enemy of consistency and purity. Graphite's chemical inertness makes it a stable, predictable heat source that will not contaminate the product or destroy itself in environments where most other materials would fail.

What Chemical Inertness Means in Practice
Chemical inertness is not an abstract property; it has direct, practical consequences for furnace operation, product quality, and equipment longevity. At the extreme temperatures where graphite elements operate, even materials considered stable can become highly reactive.
Resisting Chemical Attack
The strength of the carbon-carbon bonds in graphite's structure makes it exceptionally resistant to attack from a wide range of acids, bases, and molten metals.
Unlike metallic heating elements that can corrode or form alloys when exposed to process chemicals, graphite remains stable. This makes it the material of choice for applications involving aggressive substances.
Preventing Process Contamination
For many advanced manufacturing processes, purity is paramount. This includes applications like semiconductor crystal growth, specialized glass production, and high-purity metallurgy.
Because graphite does not react with the process atmosphere or the material being heated (the "workload"), it does not introduce impurities. The heat it provides is "clean," ensuring the final product meets strict quality specifications.
Ensuring Element Longevity
The operational lifespan of a heating element is directly tied to its ability to withstand its environment. A reactive element will degrade over time, leading to inconsistent performance and eventual failure.
Graphite's inertness means it does not corrode or wear away due to chemical reactions. This results in a significantly longer and more predictable service life, reducing downtime and maintenance costs.
The Critical Trade-off: Oxidation
While graphite is remarkably inert in many situations, it has one major vulnerability: oxygen. This limitation defines how and where graphite heating elements can be used.
The Necessity of a Controlled Atmosphere
Graphite will begin to oxidize (effectively, burn) in the presence of air at temperatures above approximately 450°C (842°F). Therefore, graphite heating elements cannot be operated in an open-air environment at high temperatures.
They must be used within a vacuum or in a furnace backfilled with an inert gas, such as argon or nitrogen. This protective atmosphere prevents oxidation and allows the element to reach temperatures far exceeding those of most metallic heaters, often over 2000°C (3632°F).
The Risk of Carburization
While graphite is non-reactive, its carbon can sometimes diffuse into the material being heated, a process known as carburization.
For certain materials, like specific steel alloys where carbon content must be meticulously controlled, this can be a disadvantage. In these cases, the process must be carefully managed, or a different type of ceramic heater (like molybdenum disilicide) may be considered.
Making the Right Choice for Your Goal
Selecting a heating element requires matching its properties to your process requirements. Graphite's inertness makes it ideal for specific, demanding scenarios.
- If your primary focus is process purity: Graphite's non-reactivity is ideal for preventing contamination in semiconductor, electronics, and high-purity metal applications.
- If your primary focus is extreme temperatures: In a vacuum or inert gas, graphite maintains its structural integrity at temperatures that would melt or destroy conventional metallic elements.
- If your primary focus is chemical resistance: Graphite excels in environments with corrosive materials or molten metals that would rapidly degrade other heaters.
Ultimately, leveraging graphite's chemical inertness allows for reliable, clean heating in some of the most challenging industrial and scientific environments imaginable.
Summary Table:
| Advantage | Practical Benefit |
|---|---|
| Resists Chemical Attack | Stable in contact with acids, bases, and molten metals. |
| Prevents Process Contamination | Delivers 'clean' heat for high-purity applications like semiconductors. |
| Ensures Element Longevity | Non-reactive nature leads to longer service life and less downtime. |
| Requires Controlled Atmosphere | Must operate in vacuum or inert gas to prevent oxidation above 450°C. |
Leverage Graphite's Superior Inertness with KINTEK Solutions
Are you tackling high-temperature processes where purity and reliability are non-negotiable? KINTEK's advanced graphite heating elements are engineered for excellence. Our in-house manufacturing and deep R&D capabilities allow us to provide robust, chemically inert solutions—including Tube, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems—that precisely match your unique experimental or production requirements.
Contact us today to discuss how our high-temperature furnace solutions can enhance your process efficiency and product quality.
Visual Guide
Related Products
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
- Silicon Carbide SiC Thermal Heating Elements for Electric Furnace
- Laboratory Muffle Oven Furnace with Bottom Lifting
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Split Multi Heating Zone Rotary Tube Furnace Rotating Tube Furnace
People Also Ask
- What is the significance of vacuum in relation to graphite components in furnaces? Prevent Oxidation for Extreme Temperatures
- What additional processes can a vacuum heat treatment furnace carry out? Unlock Advanced Material Processing
- How does vacuum heat treating affect the grain structure of metal alloys? Achieve Precise Microstructure Control
- Why is graphite cost-effective for vacuum furnaces? Maximize Long-Term ROI & Efficiency
- How does graphite contribute to energy efficiency in vacuum furnaces? Achieve Faster, More Uniform Heating


















