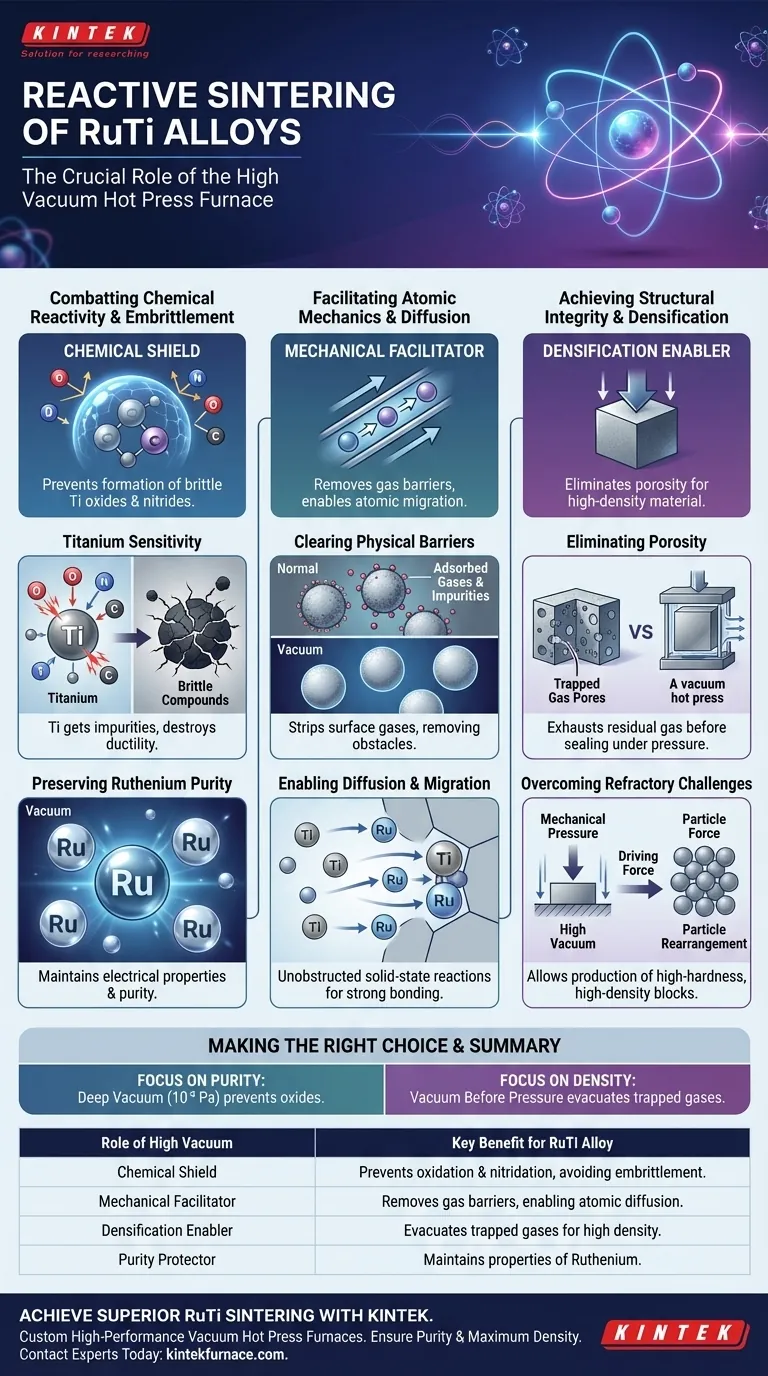
The reactive sintering of Ruthenium-Titanium (RuTi) alloys relies on a high vacuum environment primarily to counteract the extreme chemical volatility of Titanium at elevated temperatures. Without this vacuum, the Titanium component would rapidly oxidize and react with atmospheric gases, destroying the alloy's integrity, while trapped interstitial gases would physically prevent the powder particles from fusing into a dense, solid material.
Core Insight Success in sintering RuTi is not just about applying heat; it is about creating a pristine environment where atomic migration can occur without interference. The high vacuum serves a dual purpose: it acts as a chemical shield against embrittlement caused by impurities and a mechanical facilitator that removes gas barriers to ensure high-density grain bonding.

Combatting Chemical Reactivity
The Titanium Sensitivity
Titanium (Ti) is the primary driver for the necessity of high vacuum (e.g., $10^{-3}$ Pa to $5 \times 10^{-2}$ Pa). Ti is an extremely active element that acts as a "getter" for impurities at high temperatures.
Without a vacuum, Ti reacts aggressively with Oxygen, Nitrogen, and Carbon present in the air. This reaction forms brittle compounds—such as oxides and nitrides—rather than the desired RuTi intermetallic compounds.
Preserving Ruthenium Purity
While Titanium is the most sensitive component, the vacuum also protects the metallic Ruthenium (Ru). Preventing the oxidation of Ru is essential to maintain the electrical properties and chemical purity of the final alloy.
Preventing Embrittlement
The intrusion of interstitial elements like Oxygen and Nitrogen destroys the ductility of the alloy. By isolating the raw materials from these atmospheric gases, the vacuum furnace minimizes contamination. This ensures the final material retains the necessary mechanical properties, avoiding the formation of brittle inclusions that lead to structural failure.
Facilitating Atomic Mechanics
Clearing Physical Barriers
Before sintering begins, powder particles often have layers of adsorbed gases and volatile impurities on their surfaces. These layers act as physical barriers between the Ru and Ti particles.
The high vacuum environment effectively strips these adsorbed gases away. By "cleaning" the surface of the powders, the vacuum removes the obstacles that would otherwise block atomic contact.
Enabling Diffusion and Migration
Sintering relies on atoms moving across particle boundaries to bond together (atomic diffusion). Once the gaseous obstacles are removed, the barrier for this migration is lowered.
This allows for unobstructed solid-state reactions between the Ruthenium and Titanium. The result is the pure generation of RuTi phases and strong, integral bonding at the grain boundaries.
Achieving Structural Integrity
Eliminating Porosity
A major challenge in sintering is "densification"—turning loose powder into a solid block. If gas remains trapped between particles during heating, it creates closed pores, resulting in a sponge-like, low-density material.
Vacuum Hot Pressing exhausts this residual gas before the material seals up. This allows the external mechanical pressure to fully compact the material, increasing the final density of the alloy block.
Overcoming Refractory Challenges
RuTi alloys are refractory (resistant to heat) and difficult to densify using conventional methods. The combination of high vacuum and mechanical pressure creates the necessary driving force to overcome surface tension.
This promotes particle rearrangement and plastic deformation, allowing the production of high-hardness, high-density blocks that would be impossible to create in an atmospheric furnace.
Understanding the Constraints
Process Complexity and Cost
While high vacuum is necessary for quality, it introduces significant operational overhead. Achieving and maintaining vacuum levels like $10^{-3}$ Pa requires sophisticated pumping systems and immaculate seal integrity, driving up the cost of production compared to non-reactive sintering methods.
The Risk of Volatilization
There is a delicate balance in vacuum sintering. While the goal is to remove impurities, excessively high vacuum combined with extreme heat can theoretically risk volatilizing specific alloy components if not precisely controlled. However, for RuTi, the priority remains the aggressive removal of oxygen to prevent total batch failure.
Making the Right Choice for Your Goal
To optimize your sintering process, align your operational parameters with your specific quality targets:
- If your primary focus is Chemical Purity: Prioritize maintaining a deep vacuum ($10^{-3}$ Pa range) throughout the heating ramp to strictly prevent the formation of brittle Titanium oxides and nitrides.
- If your primary focus is Mechanical Density: Ensure the vacuum is fully established before applying peak mechanical pressure to guarantee all interstitial gases are evacuated, preventing trapped porosity.
Summary: The high vacuum in a hot press furnace is not merely a precaution; it is the fundamental enabler that allows reactive Titanium to bond with Ruthenium rather than reacting with the atmosphere.
Summary Table:
| Role of High Vacuum | Key Benefit for RuTi Alloy |
|---|---|
| Chemical Shield | Prevents oxidation & nitridation of Titanium, avoiding embrittlement. |
| Mechanical Facilitator | Removes gas barriers, enabling atomic diffusion and strong grain bonding. |
| Densification Enabler | Evacuates trapped gases to eliminate porosity under pressure for high density. |
| Purity Protector | Maintains the chemical and electrical properties of Ruthenium. |
Achieve Superior RuTi Alloy Sintering with KINTEK
Struggling with oxidation, porosity, or inconsistent results in your high-temperature material synthesis? The precise high-vacuum environment is the key to success.
Backed by expert R&D and manufacturing, KINTEK offers Muffle, Tube, Rotary, Vacuum, and CVD systems, including high-performance vacuum hot press furnaces. Our lab high-temp furnaces are all customizable for unique needs like reactive sintering of sensitive alloys.
Let us help you ensure chemical purity and achieve maximum density in your materials.
Contact our experts today to discuss your specific application and find the perfect furnace solution for your lab.
Visual Guide
Related Products
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- 600T Vacuum Induction Hot Press Vacuum Heat Treat and Sintering Furnace
- 9MPa Air Pressure Vacuum Heat Treat and Sintering Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Molybdenum Vacuum Heat Treat Furnace
People Also Ask
- Why is a high-vacuum environment necessary for sintering Cu/Ti3SiC2/C/MWCNTs composites? Achieve Material Purity
- Why is a vacuum environment essential for sintering Titanium? Ensure High Purity and Eliminate Brittleness
- What is the function of a vacuum sintering furnace in the SAGBD process? Optimize Magnetic Coercivity and Performance
- How do vacuum sintering and annealing furnaces contribute to the densification of NdFeB magnets?
- What is the purpose of setting a mid-temperature dwell stage? Eliminate Defects in Vacuum Sintering



















