At its core, an induction furnace can smelt any material that is electrically conductive. This includes a wide range of common metals such as steel, stainless steel, copper, and aluminum, as well as precious metals like gold and silver. The process is exceptionally clean and fast because the heat is generated directly within the material itself.
The defining characteristic of an induction furnace is its heating method. It relies on electromagnetic induction to heat materials, meaning its primary application is for melting electrically conductive metals and alloys. Non-conductive materials like ceramics can only be heated indirectly.

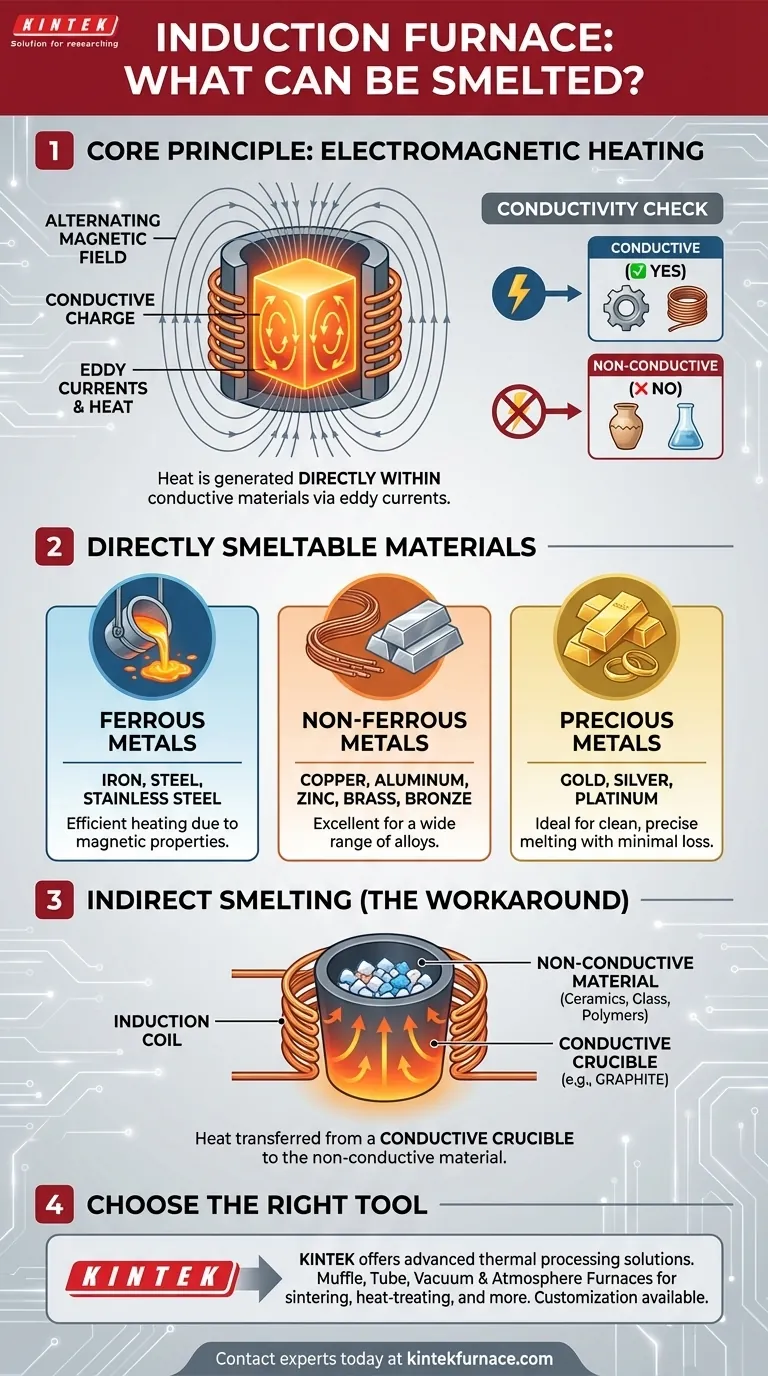
The Core Principle: Heating with Electromagnetism
An induction furnace doesn't use flames or external heating elements to melt material. Instead, it uses physics to generate heat from within the target material, which gives it unique advantages and limitations.
How Induction Generates Heat
An induction furnace uses a powerful alternating current passed through a copper coil. This creates a rapidly alternating magnetic field around the coil.
When an electrically conductive material (the "charge") is placed inside this field, the magnetic field induces powerful electrical currents within the metal. These currents, known as eddy currents, flow against the metal's natural resistance, generating intense and precise heat.
The Critical Role of Conductivity
This heating method only works if the material can conduct electricity. The ability of the material to support the flow of eddy currents is what allows it to heat up.
This is the fundamental principle that dictates what an induction furnace can and cannot melt directly.
A Breakdown of Compatible Materials
Because the primary requirement is electrical conductivity, induction furnaces are the tool of choice for a vast range of metals found in foundries and labs.
Ferrous Metals
This is the most common application. Induction furnaces are widely used for melting iron and steel, including cast iron, carbon steel, and various stainless steel alloys. The strong magnetic properties of ferrous metals make them heat very efficiently.
Non-Ferrous Metals
Metals that do not contain significant amounts of iron are also easily melted. This includes copper, aluminum, zinc, tin, and their alloys like brass and bronze.
Precious Metals
The precision, speed, and cleanliness of induction melting make it ideal for high-value materials. Gold, silver, platinum, and other platinum-group metals can be melted with minimal material loss or contamination.
Understanding the Limitations and Trade-offs
No technology is a universal solution. The primary limitation of an induction furnace is directly tied to its greatest strength: its heating mechanism.
The Inability to Heat Non-Conductive Materials
An induction furnace cannot directly heat materials that do not conduct electricity. This includes ceramics, glass, polymers, and dry aggregates. The magnetic field will pass through them without inducing any heating currents.
The Workaround: Using a Conductive Crucible
It is possible to melt or heat non-conductive materials indirectly. This is done by placing the material inside a conductive crucible, most commonly made of graphite or silicon carbide.
The induction coil heats the crucible, and the crucible then transfers its heat to the material inside through conduction and radiation. This allows the furnace to be used for a wider range of applications, but it is less efficient than direct induction heating.
When to Consider Other Furnace Types
Other furnace technologies exist for specific goals. A vacuum furnace, for example, is not defined by its heating method but by its ability to control the atmosphere. It's used for processes like sintering or heat-treating sensitive alloys where preventing oxidation is critical.
Making the Right Choice for Your Material
Your choice of furnace technology should be dictated by the material you are processing and your ultimate goal.
- If your primary focus is melting metals (ferrous, non-ferrous, or precious): An induction furnace is the ideal choice for its speed, efficiency, and cleanliness.
- If your primary focus is processing non-conductive materials (like ceramics or glass): You will need a conductive crucible within an induction furnace or a different technology like a resistance-heated box furnace.
- If your primary focus is preventing oxidation or sintering advanced materials: A vacuum furnace is specifically designed for these atmosphere-controlled processes, regardless of the heating method used inside.
Understanding the fundamental heating principle of each furnace is the key to selecting the right tool for your application.
Summary Table:
| Material Type | Examples | Can Be Melted Directly? | Notes |
|---|---|---|---|
| Ferrous Metals | Iron, Steel, Stainless Steel | Yes | Heats very efficiently due to magnetic properties. |
| Non-Ferrous Metals | Copper, Aluminum, Zinc, Brass, Bronze | Yes | Excellent for a wide range of alloys. |
| Precious Metals | Gold, Silver, Platinum | Yes | Ideal for clean melting with minimal loss. |
| Non-Conductive Materials | Ceramics, Glass, Polymers | No (Requires a conductive crucible) | Must be heated indirectly via a graphite crucible. |
Ready to Smelt Your Materials with Precision?
Understanding your material's properties is the first step. The next is choosing the right furnace technology to achieve your goals efficiently and cleanly.
KINTEK excels in providing advanced thermal processing solutions. Leveraging our exceptional R&D and in-house manufacturing, we offer a diverse range of high-temperature furnaces, including precisely controlled Muffle, Tube, and Vacuum & Atmosphere Furnaces, perfect for sintering, heat-treating, and other applications where atmosphere control is critical.
Have a unique material or a specific experimental requirement? Our strong deep customization capability allows us to tailor solutions to your exact needs.
Let's discuss your project. Contact our experts today to find the perfect furnace solution for your laboratory or production line.
Visual Guide
Related Products
- Vacuum Induction Melting Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- How has vacuum smelting impacted the development of superalloys? Unlock Higher Strength and Purity
- What are the common applications of Vacuum Induction Melting? Essential for High-Performance Metals and Alloys
- Why is a Vacuum Induction Melting (VIM) furnace essential? Unlock Purity for Aerospace and Semiconductors
- How does vacuum melting technology contribute to sustainability? Boost Durability and Recycling Efficiency
- What are some common applications of vacuum induction melting and casting (VIM&C)? Essential for Aerospace, Medical, and Nuclear Industries



















