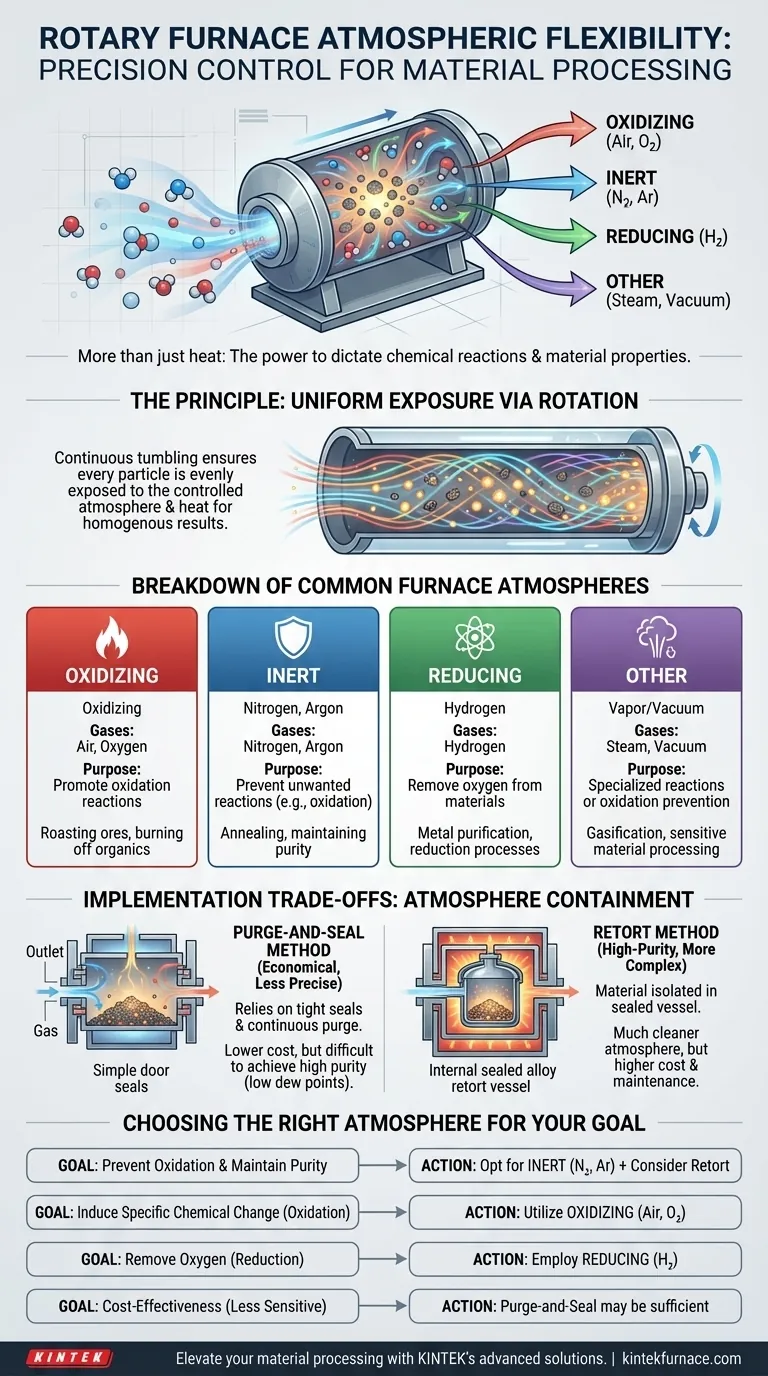
At their core, rotary furnaces are designed for impressive atmospheric flexibility. They can operate in oxidizing atmospheres like air or oxygen, inert atmospheres like nitrogen or argon, or chemically reactive atmospheres containing gases like hydrogen and steam. This control is crucial because the atmosphere directly dictates the chemical reactions that occur during heat treatment, determining whether a material is purified, oxidized, reduced, or otherwise chemically altered.
The true value of a rotary furnace isn't just its ability to heat and mix; it's the power to precisely control the chemical environment. This control is the determining factor in achieving the desired material properties, making atmosphere selection a critical process parameter.

The Principle of Atmosphere Control
The fundamental goal of atmosphere control is to manage the chemical environment surrounding the material being processed. This is often more important than the heating itself.
What is a Controlled Atmosphere?
A controlled atmosphere is a specifically blended gas mixture that displaces the ambient air within the furnace chamber. This allows for the precise management of chemical reactions at high temperatures.
By removing reactive elements like oxygen, or by intentionally introducing specific reactive gases, you can guide the material's transformation.
Why This is Critical for Material Processing
Many materials are highly reactive at elevated temperatures. Uncontrolled exposure to air can lead to oxidation, which may be undesirable and can compromise the material's purity, strength, or intended properties.
Conversely, some processes, like calcination or roasting, require oxidation. Atmosphere control gives you the power to either prevent or promote these reactions as needed.
The Role of Furnace Rotation
The continuous rotation and tilted angle of a rotary furnace are key to its effectiveness. This action constantly tumbles and mixes the material.
This tumbling ensures that every particle is uniformly exposed to the controlled atmosphere and the heat source, leading to a consistent and homogenous final product.
A Breakdown of Common Furnace Atmospheres
The choice of atmosphere is dictated entirely by the process goal. Each type serves a distinct chemical purpose.
Oxidizing Atmospheres (Air, Oxygen)
An oxidizing atmosphere is used when the goal is to intentionally react the material with oxygen.
Common applications include roasting certain ores to convert sulfides to oxides or burning off organic binders from a ceramic composite.
Inert Atmospheres (Nitrogen, Argon)
Inert atmospheres are the most common solution for preventing unwanted chemical reactions, primarily oxidation. Gases like nitrogen and argon do not readily react with other elements.
This is essential for processes like annealing, where the goal is to alter a material's physical properties through heat without changing its chemical composition.
Reducing Atmospheres (Hydrogen)
A reducing atmosphere is used to actively remove oxygen from a material. It is the chemical opposite of an oxidizing atmosphere.
Gases like hydrogen are introduced to react with and strip away oxygen atoms from metal oxides, a key step in purifying certain metals.
Other Process Atmospheres (Steam, Vacuum)
Specialized processes may use other atmospheres. Steam can be used in certain gasification or reforming reactions.
While less common in rotary designs, the principle of using a vacuum to remove all gases is another method for processing highly oxidation-sensitive materials.
Understanding the Implementation Trade-offs
The method used to contain the controlled atmosphere involves significant engineering trade-offs between cost and performance.
The Purge-and-Seal Method
This more economical approach relies on tight door seals and welded furnace casings to contain the atmosphere. Gas is continuously purged through the chamber to displace air and contaminants.
While cost-effective, this method is less precise. It can be difficult to achieve the extremely low oxygen or moisture levels (low dew points) required for highly sensitive materials.
The Retort Method
In this design, the material is placed inside a sealed alloy container, known as a retort, which is then heated externally by the furnace.
This method provides a much cleaner, more tightly controlled atmosphere because the material is isolated from the furnace's heating elements and any potential leaks. However, retorts are more expensive and require more maintenance.
Choosing the Right Atmosphere for Your Goal
Your process objective is the only factor that matters when selecting an atmosphere. Your choice will be a direct path to achieving your desired material outcome.
- If your primary focus is preventing oxidation and maintaining purity: Opt for an inert atmosphere like nitrogen or argon, and consider a retort-style furnace for the highest level of control.
- If your primary focus is inducing a specific chemical change (oxidation): Utilize an oxidizing atmosphere of air or enriched oxygen to facilitate the desired reaction.
- If your primary focus is removing oxygen from a material (reduction): Employ a reducing atmosphere containing gases like hydrogen to chemically strip oxygen from your material.
- If your primary focus is cost-effectiveness for less sensitive processes: A purge-and-seal furnace may be sufficient, but you must accept its limitations in ultimate atmosphere purity.
Ultimately, mastering atmosphere control transforms the rotary furnace from a simple heater into a precise chemical reactor.
Summary Table:
| Atmosphere Type | Key Gases | Primary Purpose | Common Applications |
|---|---|---|---|
| Oxidizing | Air, Oxygen | Promote oxidation reactions | Roasting ores, burning off organics |
| Inert | Nitrogen, Argon | Prevent unwanted reactions (e.g., oxidation) | Annealing, maintaining purity |
| Reducing | Hydrogen | Remove oxygen from materials | Metal purification, reduction processes |
| Other | Steam, Vacuum | Specialized reactions or oxidation prevention | Gasification, sensitive material processing |
Ready to elevate your material processing with precise atmosphere control? At KINTEK, we leverage exceptional R&D and in-house manufacturing to provide advanced high-temperature furnace solutions tailored to your needs. Our product line includes Rotary Furnaces, Muffle Furnaces, Tube Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems, all backed by strong deep customization capabilities to meet your unique experimental requirements. Whether you're working with metals, ceramics, or other materials, our expertise ensures optimal performance and results. Contact us today to discuss how we can help you achieve superior outcomes in your lab!
Visual Guide
Related Products
- Split Multi Heating Zone Rotary Tube Furnace Rotating Tube Furnace
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- Laboratory Vacuum Tilt Rotary Tube Furnace Rotating Tube Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- What factors should be considered when selecting a tube for a rotary tube furnace? Ensure Optimal Performance and Longevity
- What is the purpose of the rotation mechanism in a rotary tube furnace? Achieve Uniform Heating and Enhanced Process Control
- What are some industrial applications of rotary tube furnaces? Boost Your Material Processing Efficiency
- What are the main structural components of a rotary furnace? Explore Key Parts for Efficient Material Processing
- How do rotary tube furnaces achieve precise temperature control? Master Uniform Heating for Dynamic Processes



















