In analytical chemistry, the primary role of a muffle furnace is to serve as high-temperature sample processing equipment. It is essential for procedures that require heating a material in a controlled, contamination-free environment, such as determining the inorganic content of a sample for water quality, environmental, or material analysis.
The core value of a muffle furnace lies in its design: it isolates the sample from the heat source. This indirect heating prevents contamination from combustion byproducts, ensuring the integrity and accuracy of the chemical analysis that follows.

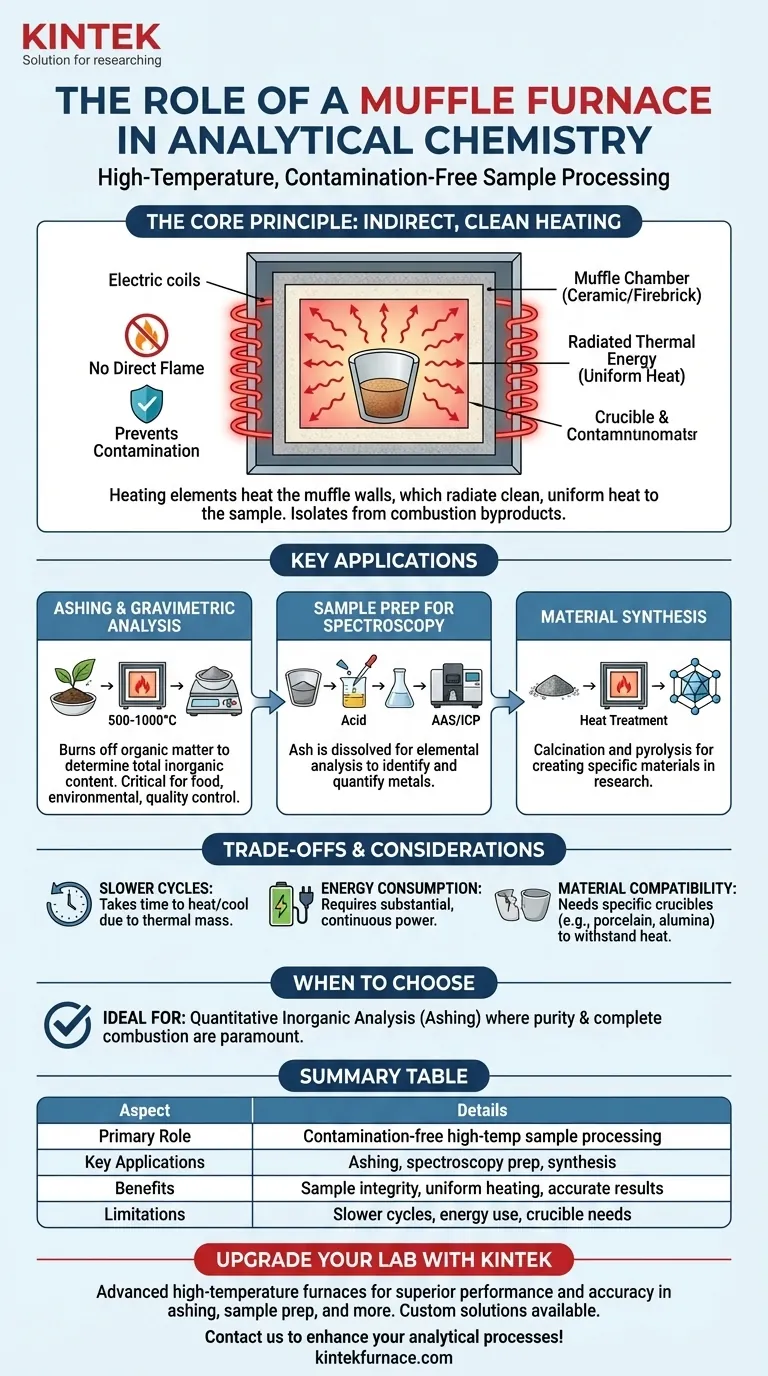
The Core Principle: Contamination-Free Heating
To understand the role of a muffle furnace, you must first understand its fundamental design principle. Unlike heating with a direct flame, a muffle furnace provides clean, uniform heat.
What "Muffle" Means
The term "muffle" refers to the furnace's inner chamber, which is typically made of high-temperature firebrick or ceramic insulation. This chamber separates the material being heated from the actual heating elements.
The insulating material acts as a barrier, or a muffle, preventing direct contact and stopping heat from escaping the cabinet.
How Indirect Heating Works
The electric coils heat the walls of the muffle chamber, not the sample itself. These superheated walls then radiate thermal energy (through convection and blackbody radiation) evenly throughout the chamber.
This process heats the sample uniformly from all sides without exposing it to flames, electrical arcs, or the byproducts of fuel combustion.
The Benefit of Purity
In analytical chemistry, even microscopic contamination can invalidate results. Direct heating with a gas flame can deposit carbon (soot) or other chemical residues onto a sample.
A muffle furnace eliminates this risk entirely. The only thing the sample is exposed to is heat and the atmosphere inside the chamber (typically air), ensuring any changes in its mass or composition are due solely to the intended thermal process.
Key Applications in Analytical Chemistry
This principle of pure, high-temperature heating makes the muffle furnace indispensable for several key analytical techniques.
Ashing and Gravimetric Analysis
The most common application is ashing. This is a method used to determine the total inorganic mineral content of a sample.
The sample (e.g., food, soil, plastic, wastewater sludge) is placed in a crucible and heated to a very high temperature, often 500-1000°C. This completely burns off all organic matter, leaving behind only the non-combustible inorganic ash.
By weighing the sample before and after ashing, analysts can precisely calculate the percentage of ash, a critical parameter in food science, environmental compliance, and quality control.
Sample Preparation for Spectroscopy
Ashing is often just the first step in a more complex analysis. The resulting inorganic ash can be dissolved in acid.
This solution is then ready for analysis by techniques like Atomic Absorption Spectroscopy (AAS) or Inductively Coupled Plasma (ICP) to identify and quantify the specific metallic elements present.
High-Temperature Material Synthesis
While less common in routine analysis, muffle furnaces are also used in materials chemistry to synthesize or treat materials.
Processes like calcination (heating to drive off volatile substances like water or CO2) and pyrolysis (thermal decomposition in the absence of oxygen) are performed to create specific crystalline structures or novel materials for research.
Understanding the Trade-offs
While essential, a muffle furnace is not the right tool for every heating task. It comes with specific operational characteristics and limitations.
Slower Heating and Cooling Cycles
Because the furnace works by heating a large, insulated thermal mass, it takes a significant amount of time to reach its target temperature. Likewise, cooling down can take several hours. This makes it unsuitable for processes requiring rapid temperature changes.
Energy Consumption
Maintaining temperatures of several hundred degrees Celsius requires a substantial and continuous input of electrical energy. This is a practical consideration for any laboratory's operational budget.
Material Compatibility
The crucible or container holding the sample must be able to withstand the extreme temperatures without melting, cracking, or reacting with the sample. This typically requires crucibles made of porcelain, high-purity alumina, or, for highly corrosive samples, platinum.
Making the Right Choice for Your Goal
Ultimately, the decision to use a muffle furnace depends entirely on the analytical objective.
- If your primary focus is quantitative analysis of inorganic content (ashing): The muffle furnace is non-negotiable, as sample purity and complete combustion are paramount for accurate results.
- If your primary focus is rapid qualitative testing or simple drying: A laboratory oven, hot plate, or even a direct flame may be a more efficient and practical choice.
- If your primary focus is synthesis under a controlled atmosphere (e.g., nitrogen or argon): A specialized tube furnace, which can be purged with inert gas, would be more appropriate than a standard muffle furnace.
Understanding the principle of indirect, contamination-free heat is the key to knowing when and why to deploy this critical analytical tool.
Summary Table:
| Aspect | Details |
|---|---|
| Primary Role | High-temperature sample processing for contamination-free heating in analytical chemistry |
| Key Applications | Ashing, gravimetric analysis, sample preparation for spectroscopy (e.g., AAS, ICP), material synthesis (calcination, pyrolysis) |
| Benefits | Prevents contamination, ensures sample integrity, uniform heating, accurate results |
| Limitations | Slower heating/cooling cycles, high energy consumption, requires compatible crucibles (e.g., porcelain, alumina) |
| Ideal For | Quantitative inorganic analysis, where purity and complete combustion are critical |
Upgrade Your Laboratory with KINTEK's Advanced High-Temperature Furnaces
Leveraging exceptional R&D and in-house manufacturing, KINTEK provides diverse laboratories with advanced high-temperature furnace solutions. Our product line, including Muffle, Tube, Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems, is complemented by our strong deep customization capability to precisely meet unique experimental requirements.
Whether you need reliable ashing for gravimetric analysis or contamination-free heating for sample prep, our furnaces deliver superior performance and accuracy. Contact us today to discuss how we can enhance your analytical processes and achieve your research goals!
Visual Guide
Related Products
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- Multi Zone Laboratory Quartz Tube Furnace Tubular Furnace
People Also Ask
- Why is re-calcination in a muffle furnace necessary for photocatalysts? Restore Efficiency via Thermal Oxidation
- What is the function of a muffle furnace in SiCf/Al-Mg pretreatment? Optimize Fiber Bonding with Thermal De-sizing
- What is the core function of a muffle furnace in CuO nanoparticle synthesis? Achieve Precision Calcination
- What is the core role of a muffle furnace in the synthesis of calcium oxide from eggshells? Achieve High-Purity CaO
- What is the primary function of a muffle furnace in iron-modified activated carbon prep? Optimize Adsorption Sites



















