In the pharmaceutical industry, a muffle furnace serves as a critical tool for high-temperature material analysis. Its primary functions are to determine the non-combustible or inorganic content of a sample through a process called ashing, to pretreat samples for further analysis, and to conduct specific quality control tests on raw materials and final drug products.
The core value of a muffle furnace in pharmaceuticals is its ability to provide a precisely controlled, contamination-free, high-temperature environment. This allows for the accurate determination of inorganic content, a fundamental step in ensuring the purity, safety, and consistency of pharmaceutical substances.

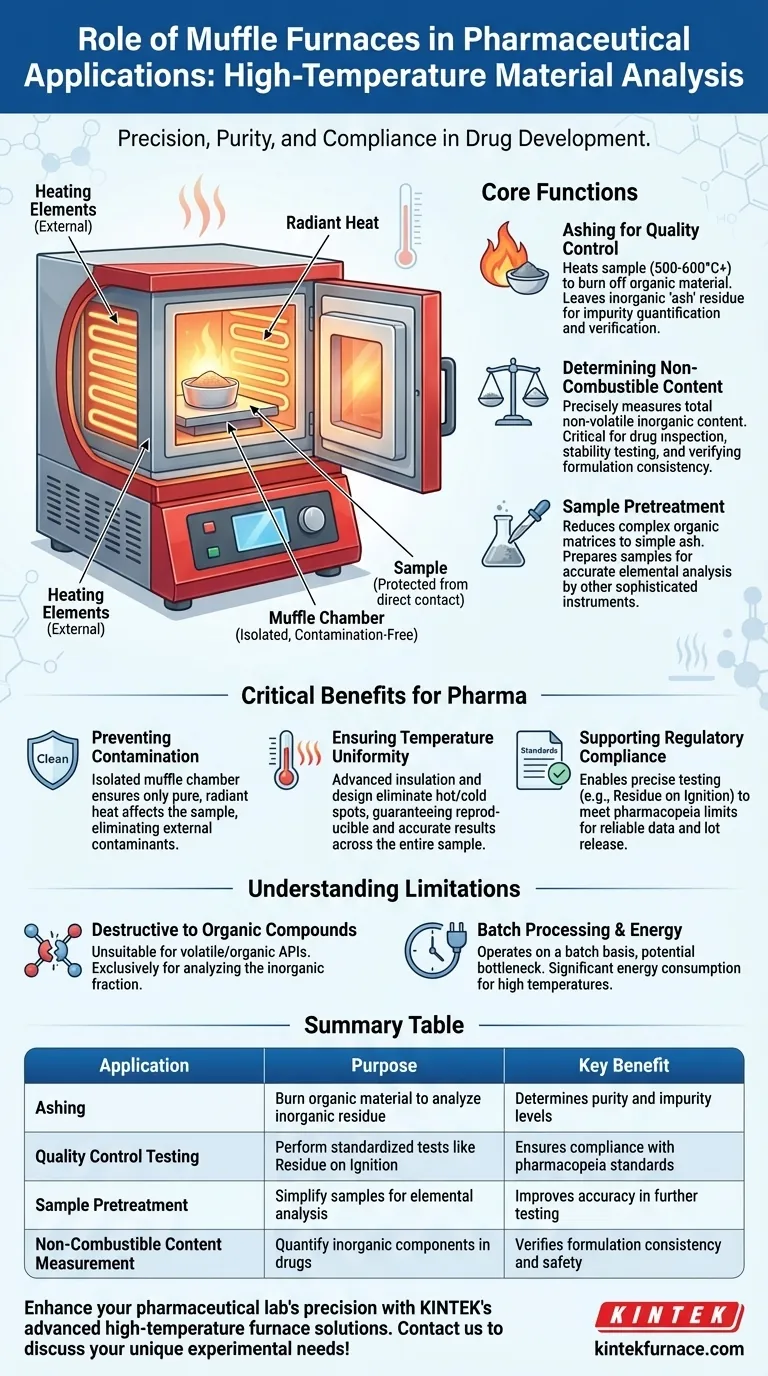
The Core Function: High-Temperature Sample Analysis
A muffle furnace is essentially a high-temperature oven, but with a key distinction. The term "muffle" refers to the fact that the sample is isolated from the heating elements and any combustion byproducts, ensuring the heating process itself does not introduce contaminants.
Ashing for Quality Control
The most common application in pharmaceuticals is ashing. This process involves heating a sample to a high temperature (often 500-600°C or higher) to completely burn away all organic and volatile substances.
What remains is the ash, which consists of the inorganic, non-combustible components. Analyzing this residue is a cornerstone of quality control, helping to quantify inorganic impurities or verify the concentration of an inorganic active ingredient.
Determining Non-Combustible Content
Beyond just impurities, the furnace is used to precisely measure the total non-volatile content of a substance. This is a critical parameter in drug inspections and stability testing.
This process helps confirm that a drug formulation contains the correct amount of its intended inorganic components and has not been contaminated or degraded.
Sample Pretreatment for Further Analysis
Sometimes, a complex sample must be simplified before it can be analyzed by other sophisticated instruments, such as those used for elemental analysis.
The muffle furnace acts as a pretreatment tool, reducing a complex organic matrix to a simple inorganic ash. This prepared sample is then easier to dissolve and analyze, leading to more accurate measurements of specific elements.
Why a Muffle Furnace is Critical for Pharma
The pharmaceutical environment demands precision, purity, and reproducibility. The design of a modern muffle furnace directly supports these requirements.
Preventing Contamination
The isolated "muffle" chamber is paramount. By separating the sample from any flames or electrical heating elements, it guarantees that the only thing affecting the sample is pure, radiant heat. This prevents contamination that could compromise the integrity of a sensitive test.
Ensuring Temperature Uniformity
Modern furnaces utilize advanced insulation and strategically placed heating elements to ensure uniform heat distribution. This eliminates hot or cold spots within the chamber, ensuring the entire sample is treated at the exact same temperature. This uniformity is crucial for achieving reproducible and accurate results.
Supporting Regulatory Compliance
Many pharmacopeias and regulatory bodies outline specific, standardized tests for drug substances, including limits for "Residue on Ignition" or "Sulphated Ash."
A muffle furnace provides the controlled environment needed to perform these tests exactly as specified, generating the reliable data required for regulatory submissions and lot release.
Understanding the Trade-offs and Limitations
While essential, a muffle furnace is a specific tool with clear limitations that must be understood.
Destructive to Organic Compounds
The furnace's primary function is to eliminate organic material. Therefore, it is completely unsuitable for analyzing any volatile or organic compounds, including most active pharmaceutical ingredients (APIs). It is a tool exclusively for analyzing the inorganic fraction of a sample.
Batch Processing Creates Bottlenecks
Muffle furnaces operate on a batch basis. A sample is loaded, heated for a set period, cooled, and then removed. This process cannot be run continuously and can become a bottleneck in high-throughput quality control labs.
Significant Energy Consumption
Reaching and maintaining temperatures of 600°C or higher requires a substantial amount of electrical energy. This is a practical operational cost that labs must factor into their budget and workflow planning.
Making the Right Choice for Your Goal
The application of a muffle furnace should be directly tied to your analytical objective.
- If your primary focus is quality control: Use the furnace to perform "Residue on Ignition" tests to quantify inorganic impurities in raw materials or final products, ensuring they meet regulatory specifications.
- If your primary focus is formulation development: Use the furnace to accurately determine the concentration of inorganic excipients or active ingredients within your formulation.
- If your primary focus is analytical testing: Use the furnace as a sample preparation step to reduce a complex drug matrix to simple ash before performing elemental analysis with other instruments.
Ultimately, the muffle furnace empowers pharmaceutical scientists to verify the fundamental composition of a substance, ensuring the safety and efficacy of medicines.
Summary Table:
| Application | Purpose | Key Benefit |
|---|---|---|
| Ashing | Burn organic material to analyze inorganic residue | Determines purity and impurity levels |
| Quality Control Testing | Perform standardized tests like Residue on Ignition | Ensures compliance with pharmacopeia standards |
| Sample Pretreatment | Simplify samples for elemental analysis | Improves accuracy in further testing |
| Non-Combustible Content Measurement | Quantify inorganic components in drugs | Verifies formulation consistency and safety |
Enhance your pharmaceutical lab's precision and compliance with KINTEK's advanced high-temperature furnace solutions. Leveraging exceptional R&D and in-house manufacturing, we offer a diverse product line—including Muffle, Tube, Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems—with strong deep customization to meet your unique experimental needs. Ensure contamination-free analysis and reliable results—contact us today to discuss how our solutions can support your quality control and regulatory goals!
Visual Guide
Related Products
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- Multi Zone Laboratory Quartz Tube Furnace Tubular Furnace
People Also Ask
- What role does a muffle furnace play in the preparation of MgO support materials? Master Catalyst Activation
- What environmental conditions are critical for SiOC ceramicization? Master Precise Oxidation & Thermal Control
- What is the primary function of a muffle furnace for BaTiO3? Master High-Temp Calcination for Ceramic Synthesis
- How does a laboratory muffle furnace facilitate the biomass carbonization process? Achieve Precise Biochar Production
- What is the role of a muffle furnace in the study of biochar regeneration and reuse? Unlock Sustainable Water Treatment



















