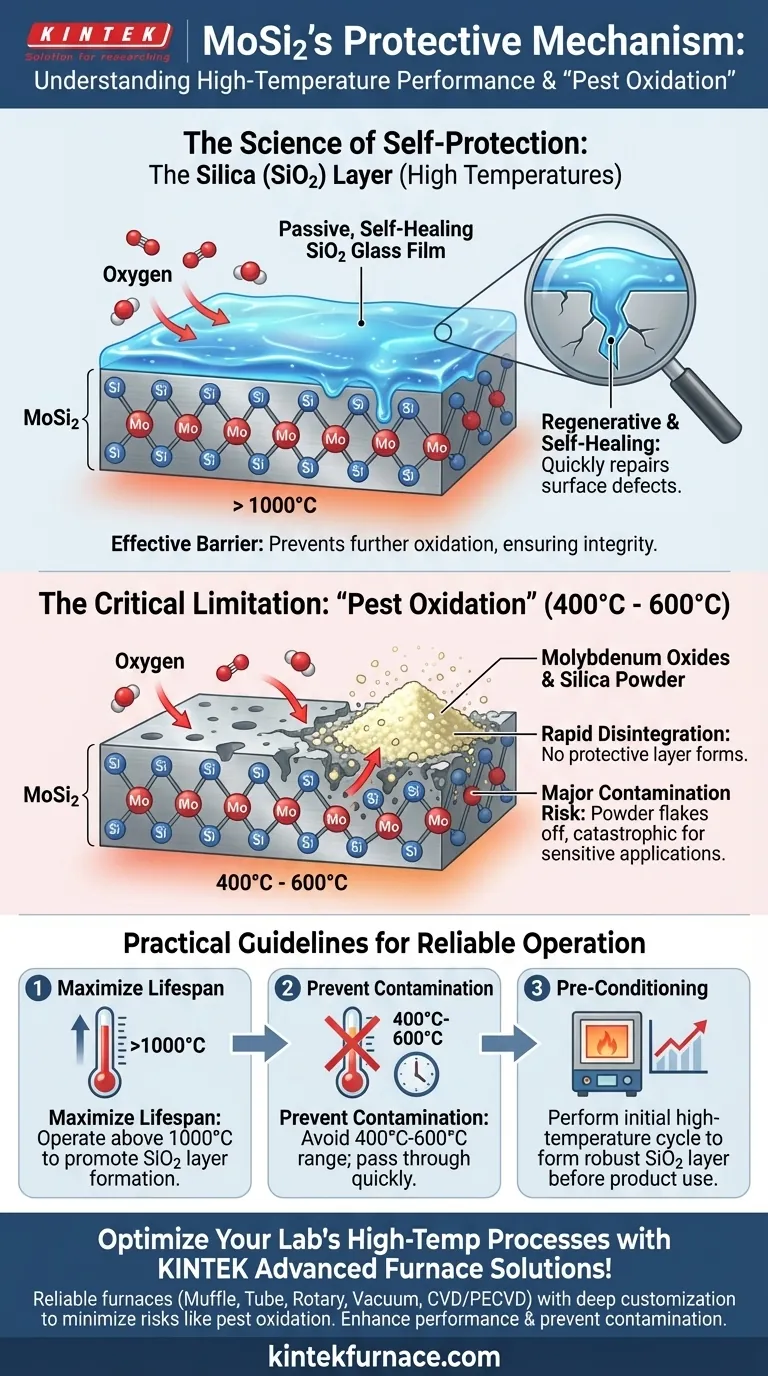
In an oxidizing atmosphere, Molybdenum Disilicide (MoSi2) protects itself by forming a passive, self-healing layer of pure silica (SiO2) glass on its surface. This regenerative film acts as a highly effective barrier, preventing further oxidation of the underlying material and ensuring its integrity at extreme temperatures.
MoSi2's remarkable performance as a high-temperature material stems from its ability to form a protective silica layer. However, understanding its one critical weakness—a low-temperature "pest oxidation"—is essential for reliable operation and preventing product contamination.

The Science of Self-Protection: The Silica (SiO2) Layer
The protective mechanism is not simply a coating applied during manufacturing; it is an active, dynamic process that occurs during operation.
How the Protective Layer Forms
When MoSi2 is heated in the presence of oxygen, the silicon within the compound readily reacts with atmospheric oxygen. This reaction forms a thin, dense, and highly stable layer of silica (SiO2), which is essentially a type of glass.
This silica film is non-porous and adheres strongly to the MoSi2 substrate, creating a formidable barrier against further oxygen ingress.
A Regenerative "Glass" Film
The most valuable characteristic of this SiO2 layer is its self-healing or regenerative nature. The layer behaves like a viscous fluid at high temperatures.
If a micro-crack or other surface defect develops, the underlying MoSi2 is immediately exposed to the oxidizing atmosphere. This exposure triggers a rapid, localized reaction that forms new SiO2, effectively "healing" the breach and restoring the protective shield.
Why This Matters for High-Temperature Use
This continuous self-repair mechanism is why MoSi2 heating elements have such a long lifespan and stable performance in air and other oxidizing environments, far surpassing many metallic or silicon carbide elements under similar conditions.
Understanding the Critical Limitation: "Pest Oxidation"
While exceptionally robust at high temperatures, the protective mechanism of MoSi2 has a well-documented vulnerability at lower temperatures.
The Problem at Low Temperatures
In a temperature range of approximately 400°C to 600°C, a different and destructive form of oxidation known as pest oxidation can occur.
Instead of forming a dense, protective glass layer, the material rapidly disintegrates into a yellowish powder composed of molybdenum oxides and silica. This process is accelerated by the material's inherent porosity.
The Consequence of "Pest": Contamination
This pest reaction does not form a protective barrier. The resulting powder can easily flake off the element's surface.
While this may not cause immediate element failure, it is a significant source of product contamination. In sensitive applications like semiconductor processing or ceramic firing, this contamination can be catastrophic to the final product.
The Practical Mandate: Avoid the Pest Zone
Because of the risk of pest oxidation, continuous operation of MoSi2 elements within the 400°C to 600°C range must be strictly avoided. Heating and cooling cycles should be programmed to pass through this temperature zone as quickly as possible.
A Guideline for Using MoSi2 Elements
Understanding this dual behavior is key to leveraging the material's strengths while mitigating its risks.
- If your primary focus is maximizing element lifespan: Ensure a stable oxidizing atmosphere above 1000°C to promote the formation and regeneration of the protective SiO2 glass layer.
- If your primary focus is preventing product contamination: You must design heating cycles to move rapidly through the 400°C-600°C range to prevent the formation of pest-related powder.
- If you are commissioning a new furnace: Perform an initial high-temperature cycle in air to "pre-condition" the elements, allowing them to form a robust initial SiO2 layer before any product is introduced.
By managing the thermal profile to account for these distinct behaviors, you can ensure reliable and long-lasting performance from your MoSi2 components.
Summary Table:
| Protective Mechanism | Key Details | Temperature Range |
|---|---|---|
| Silica (SiO2) Layer Formation | Forms a dense, non-porous barrier that prevents oxygen ingress and self-heals cracks. | Above 1000°C |
| Pest Oxidation | Rapid disintegration into powder, causing contamination; avoid prolonged exposure. | 400°C to 600°C |
Optimize your lab's high-temperature processes with KINTEK's advanced furnace solutions! Leveraging exceptional R&D and in-house manufacturing, we provide diverse laboratories with reliable high-temperature furnaces like Muffle, Tube, Rotary, Vacuum & Atmosphere, and CVD/PECVD Systems. Our strong deep customization capability ensures precise solutions for your unique experimental needs, minimizing risks like pest oxidation. Contact us today to enhance performance and prevent contamination!
Visual Guide
Related Products
- Molybdenum Vacuum Heat Treat Furnace
- Silicon Carbide SiC Thermal Heating Elements for Electric Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Laboratory Muffle Oven Furnace with Bottom Lifting
- Split Multi Heating Zone Rotary Tube Furnace Rotating Tube Furnace
People Also Ask
- What are the benefits of using a high-temperature vacuum furnace for the annealing of ZnSeO3 nanocrystals?
- Why is a high-vacuum environment necessary for sintering Cu/Ti3SiC2/C/MWCNTs composites? Achieve Material Purity
- What is the role of vacuum pumps in a vacuum heat treatment furnace? Unlock Superior Metallurgy with Controlled Environments
- What tasks does a high-temperature vacuum sintering furnace perform for PEM magnets? Achieve Peak Density
- What is the purpose of a 1400°C heat treatment for porous tungsten? Essential Steps for Structural Reinforcement



















