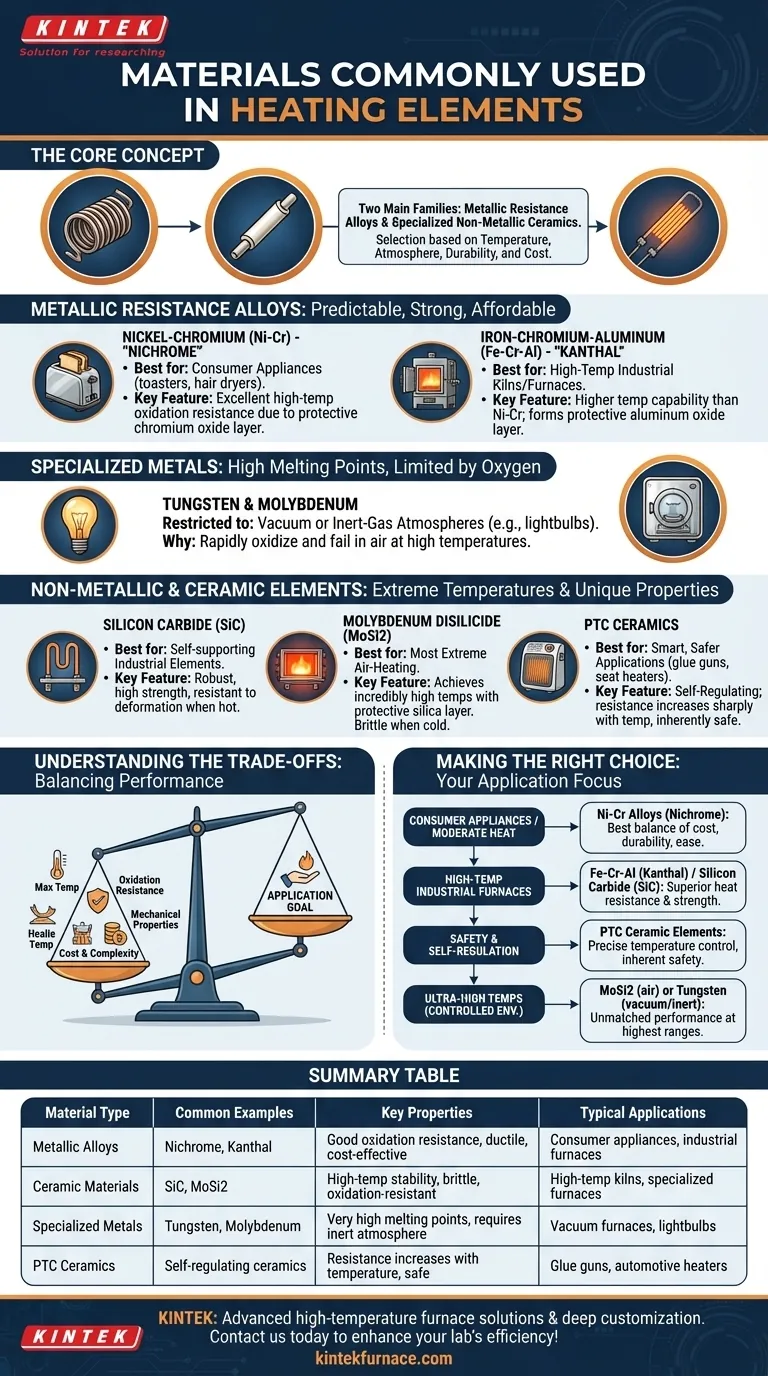
At their core, heating elements are most commonly made from either metallic resistance alloys or specialized non-metallic ceramic materials. The most prevalent metallic options are nickel-chromium (Ni-Cr) alloys, known as Nichrome, and iron-chromium-aluminum (Fe-Cr-Al) alloys, such as Kanthal. For very high-temperature or specialized applications, ceramic materials like silicon carbide (SiC) and molybdenum disilicide (MoSi2) are used.
The selection of a heating element material is not about finding a single "best" option. It is a precise engineering choice dictated by the required operating temperature, the surrounding atmosphere, and mechanical durability, all balanced against cost.

The Two Families of Heating Element Materials
Nearly all modern heating elements fall into one of two categories: metallic alloys, which are workhorses for a vast range of temperatures, and non-metallic ceramics, which are reserved for more extreme or specialized conditions.
Metallic Resistance Alloys
These materials are valued for their predictable resistance, strength, and relative affordability. They work by resisting the flow of electricity, which generates heat.
Nickel-Chromium (Ni-Cr) Alloys
Commonly known by the brand name Nichrome, this is the most recognized heating element material. It is the standard for countless consumer appliances like toasters, hair dryers, and space heaters. Its key advantage is its excellent resistance to high-temperature oxidation. When heated, it forms a protective outer layer of chromium oxide that prevents the underlying metal from degrading, giving it a long service life in air.
Iron-Chromium-Aluminum (Fe-Cr-Al) Alloys
Often referred to by the brand name Kanthal, these alloys represent the next step up in temperature capability. They can operate at higher temperatures than most Ni-Cr alloys, making them a top choice for industrial applications like high-temperature kilns and furnaces. Like Nichrome, they also form a protective oxide layer (aluminum oxide, in this case) that contributes to their durability.
Specialized Metals (Tungsten & Molybdenum)
Metals like Tungsten (W) and Molybdenum (Mo) have exceptionally high melting points. However, they oxidize and fail very quickly in the presence of oxygen at high temperatures. Because of this, they are restricted to specialized applications that operate in a vacuum or a controlled, inert-gas atmosphere. The filament in an incandescent lightbulb is a classic example of a tungsten heating element.
Non-Metallic and Ceramic Elements
When temperatures push beyond the limits of conventional alloys or when unique properties are needed, engineers turn to ceramics and composites.
Silicon Carbide (SiC)
Silicon Carbide is a robust ceramic material that can operate at very high temperatures. Unlike many metals, it is very strong and resistant to deformation or "creep" when hot. This makes it ideal for self-supporting elements in industrial furnaces and kilns where mechanical stability is crucial.
Molybdenum Disilicide (MoSi2)
For the most extreme air-heating applications, Molybdenum Disilicide is the material of choice. It can achieve incredibly high temperatures while also forming a protective silica layer that resists oxidation. Its primary drawback is that it is very brittle at room temperature, which requires careful handling and installation.
Positive Temperature Coefficient (PTC) Ceramics
PTC ceramics are "smart" materials. Their electrical resistance increases sharply once they reach a specific design temperature. This unique property makes them self-regulating and inherently safe, as they naturally limit their heat output and prevent overheating. They are commonly found in smaller, safer heating applications like glue guns and some automotive seat heaters.
Understanding the Trade-offs
Choosing the right material requires balancing performance characteristics. The ideal choice for a toaster is entirely wrong for an industrial furnace.
Maximum Operating Temperature
This is the primary constraint. Fe-Cr-Al alloys generally operate at higher temperatures than Ni-Cr alloys. Ceramic elements like SiC and MoSi2 push the boundaries far beyond what even the best alloys can achieve.
Resistance to Oxidation
The ability to survive in open air at high temperatures is critical. Both Ni-Cr and Fe-Cr-Al alloys excel here because they form their own protective oxide layers. Tungsten, by contrast, has a very high melting point but fails catastrophically in air, limiting its use.
Mechanical Properties
Material behavior at different temperatures matters. MoSi2 is capable of extreme heat but is brittle when cold, complicating system design and maintenance. Ni-Cr is ductile and easily formed into coils, which is perfect for compact appliances.
Cost and Complexity
Common alloys like Nichrome are widespread, easy to manufacture, and cost-effective. Advanced ceramics like MoSi2 and specialized metals like Tungsten are significantly more expensive and require more complex system designs (e.g., vacuum or inert atmospheres) to function properly.
Making the Right Choice for Your Application
Your final selection depends entirely on your specific goal. The material must fit the operational demands of the system.
- If your primary focus is consumer appliances or moderate heat: Ni-Cr alloys (Nichrome) offer the best balance of cost, durability, and ease of manufacturing.
- If your primary focus is high-temperature industrial furnaces: Fe-Cr-Al alloys (Kanthal) and Silicon Carbide (SiC) are the standard choices for their superior heat resistance and strength.
- If your primary focus is safety and self-regulation: PTC ceramic elements are the ideal solution for applications where precise temperature control and inherent safety are top priorities.
- If your primary focus is ultra-high temperatures in a controlled environment: Molybdenum Disilicide (MoSi2) for air, or Tungsten for vacuum/inert gas, are necessary for their unmatched performance at the highest thermal ranges.
Ultimately, selecting the right heating element material is a direct function of balancing your temperature requirements against the operating environment and budget.
Summary Table:
| Material Type | Common Examples | Key Properties | Typical Applications |
|---|---|---|---|
| Metallic Alloys | Nichrome (Ni-Cr), Kanthal (Fe-Cr-Al) | Good oxidation resistance, ductile, cost-effective | Consumer appliances, industrial furnaces |
| Ceramic Materials | Silicon Carbide (SiC), Molybdenum Disilicide (MoSi2) | High-temperature stability, brittle, oxidation-resistant | High-temperature kilns, specialized furnaces |
| Specialized Metals | Tungsten, Molybdenum | Very high melting points, requires inert atmosphere | Vacuum furnaces, lightbulbs |
| PTC Ceramics | Self-regulating ceramics | Resistance increases with temperature, safe | Glue guns, automotive heaters |
Struggling to choose the right heating element for your lab's unique needs? KINTEK leverages exceptional R&D and in-house manufacturing to provide advanced high-temperature furnace solutions tailored for diverse laboratories. Our product line includes Muffle, Tube, Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems, complemented by strong deep customization capabilities to precisely meet your experimental requirements. Contact us today to enhance your lab's efficiency and performance with our expert solutions!
Visual Guide
Related Products
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- Multi Zone Laboratory Quartz Tube Furnace Tubular Furnace
People Also Ask
- Can a muffle furnace be used for pyrolysis? Unlock Precise Thermal Decomposition
- How do you prevent maintenance on a muffle furnace? Extend Lifespan with Proactive Care
- What is the role of a muffle furnace in the study of biochar regeneration and reuse? Unlock Sustainable Water Treatment
- What is the role of a muffle furnace in the synthesis of water-soluble Sr3Al2O6? Precision in SAO Production
- What environmental conditions are critical for SiOC ceramicization? Master Precise Oxidation & Thermal Control



















