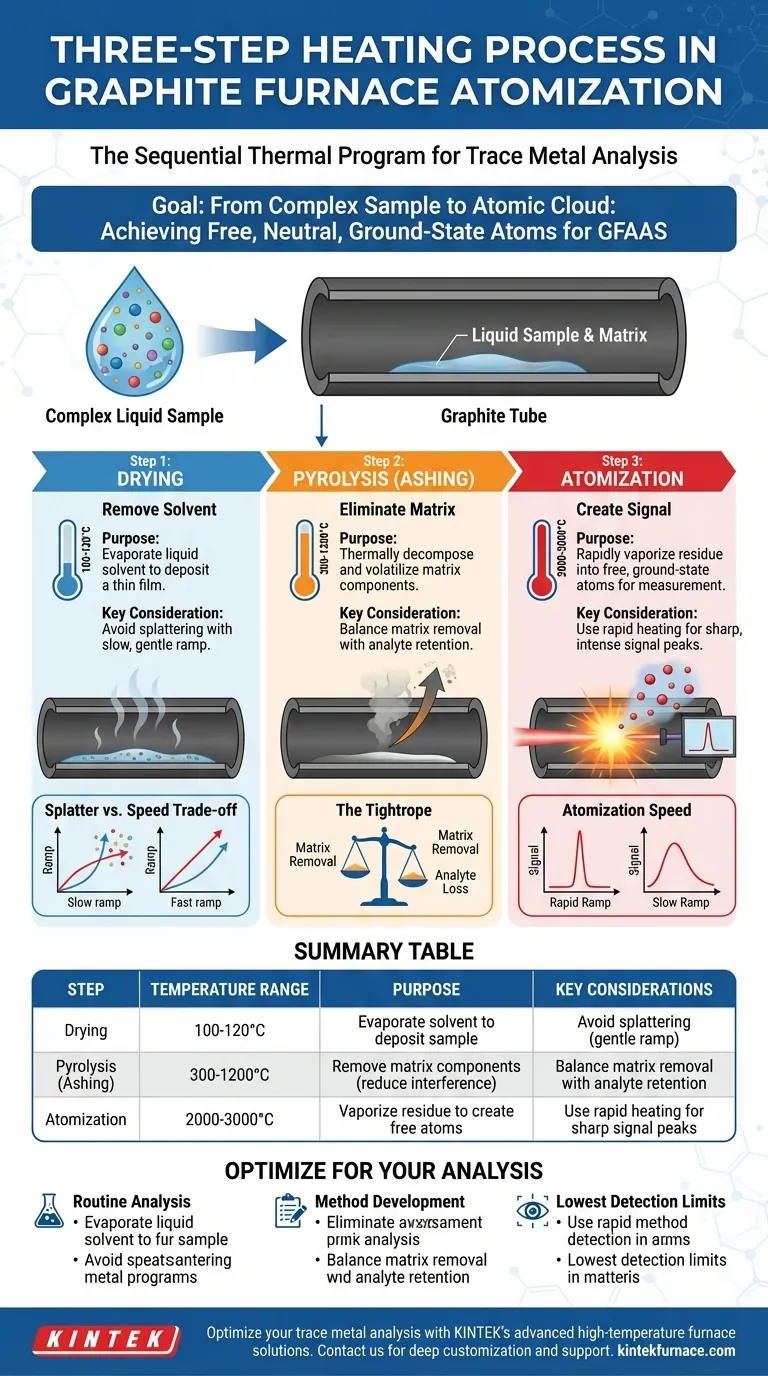
The three-step heating process in graphite furnace atomization is a sequential thermal program designed to convert a liquid sample into a cloud of free atoms for measurement. It consists of a low-temperature drying stage, a medium-temperature pyrolysis (or ashing) stage, and a high-temperature atomization stage.
The core challenge in trace metal analysis is isolating the element of interest from a complex sample matrix. The three-step heating process is not just about heating; it's a systematic purification method that occurs inside the instrument before the actual measurement, ensuring that the final signal comes only from your target analyte.

The Goal: From Complex Sample to Atomic Cloud
Graphite Furnace Atomic Absorption Spectroscopy (GFAAS) is an incredibly sensitive technique for measuring trace elements. Its power comes from its ability to detect the light absorbed by a very small number of atoms.
The fundamental requirement is that these atoms must be in a free, neutral, ground-state form, floating as a vapor inside the graphite tube. The three-step heating program is the carefully controlled process designed to achieve this state repeatably and accurately.
Step 1: Drying - Removing the Solvent
The first step involves gently heating the graphite tube to a temperature slightly above the boiling point of the solvent, typically around 100-120°C. This stage can last from several seconds to a minute.
The sole purpose of this step is to evaporate the liquid solvent (usually water or a dilute acid) in a slow, controlled manner. Proper drying deposits the sample analyte and its surrounding matrix as a thin, even film on the bottom of the furnace.
Step 2: Pyrolysis (Ashing) - Eliminating the Matrix
This is often the most critical stage for method development. The furnace temperature is raised significantly, typically to 300-1200°C, to thermally decompose and remove the bulk of the sample matrix.
The goal is to volatilize the matrix components (salts, organic matter) while ensuring the element of interest remains behind. By removing these interfering substances, this step dramatically reduces background noise and potential chemical interferences during the final measurement.
Step 3: Atomization - Creating the Signal
In the final step, the furnace is heated as rapidly as possible to a very high temperature, usually between 2000°C and 3000°C. This process takes only a few milliseconds to seconds.
This intense burst of energy instantly vaporizes the remaining residue and provides enough thermal energy to break any remaining chemical bonds. This creates the transient cloud of free, ground-state atoms that can absorb light from the source lamp, generating the analytical signal.
Understanding the Trade-offs and Pitfalls
Optimizing the heating program is a balancing act. Errors in any step can lead to inaccurate or imprecise results.
The Drying Dilemma: Splatter vs. Speed
If the drying ramp is too aggressive or the final temperature is too high, the solvent can boil violently. This causes sample splattering, leading to a physical loss of analyte and poor reproducibility. A slow, gentle ramp is safer but increases analysis time.
The Pyrolysis Tightrope: Removing Matrix vs. Losing Analyte
This is the most challenging trade-off. You must set the pyrolysis temperature high enough to remove as much of the interfering matrix as possible.
However, if the temperature is too high for your specific analyte, the analyte itself will begin to volatilize and be lost before the atomization step. This results in a signal that is artificially low. Finding the maximum possible pyrolysis temperature without losing the analyte is key to a robust method.
Atomization Speed: Capturing a Sharp Peak
The signal in GFAAS is a transient peak. For the best sensitivity and precision, the atomization step must be as fast as possible.
A rapid temperature ramp generates a dense, concentrated cloud of atoms, producing a sharp, narrow, and intense peak. A slow ramp allows atoms to diffuse out of the light path over a longer period, resulting in a low, broad signal that is difficult to measure accurately.
Optimizing the Program for Your Analysis
Understanding this three-step process empowers you to troubleshoot problems and develop robust analytical methods.
- If your primary focus is routine analysis with a known matrix: Follow the validated method's temperature program, but use this knowledge to diagnose issues like poor precision (check drying) or low recovery (check pyrolysis temperature).
- If your primary focus is method development for a new analyte: Carefully create a pyrolysis curve, testing progressively higher temperatures to find the ideal point that maximizes matrix removal without causing premature analyte loss.
- If your primary focus is achieving the lowest possible detection limits: Meticulous optimization of the pyrolysis step is non-negotiable, as minimizing background absorption from residual matrix is often the factor limiting sensitivity.
Mastering the logic of this thermal program is the foundation for achieving accurate and reliable trace element analysis.
Summary Table:
| Step | Temperature Range | Purpose | Key Considerations |
|---|---|---|---|
| Drying | 100-120°C | Evaporate solvent to deposit sample | Avoid splattering by using a slow, gentle ramp |
| Pyrolysis (Ashing) | 300-1200°C | Remove matrix components to reduce interference | Balance matrix removal with analyte retention |
| Atomization | 2000-3000°C | Vaporize residue to create free atoms for measurement | Use rapid heating for sharp, intense signal peaks |
Optimize your trace metal analysis with KINTEK's advanced high-temperature furnace solutions. Leveraging exceptional R&D and in-house manufacturing, we provide Muffle, Tube, Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems. Our strong deep customization capability ensures precise alignment with your unique experimental needs, enhancing accuracy and efficiency in your lab. Contact us today to discuss how we can support your analytical goals!
Visual Guide
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Vertical Laboratory Quartz Tube Furnace Tubular Furnace
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
- Split Multi Heating Zone Rotary Tube Furnace Rotating Tube Furnace
People Also Ask
- What safety measures are essential when operating a lab tube furnace? A Guide to Preventing Accidents
- How does a vertical tube furnace achieve precise temperature control? Unlock Superior Thermal Stability for Your Lab
- What safety and reliability features are incorporated into a vertical tube furnace? Ensuring Safe, Consistent High-Temp Processing
- How is a Vertical Tube Furnace used for fuel dust ignition studies? Model Industrial Combustion with Precision
- How is a high-temperature tube furnace utilized in the synthesis of MoO2/MWCNTs nanocomposites? Precision Guide



















