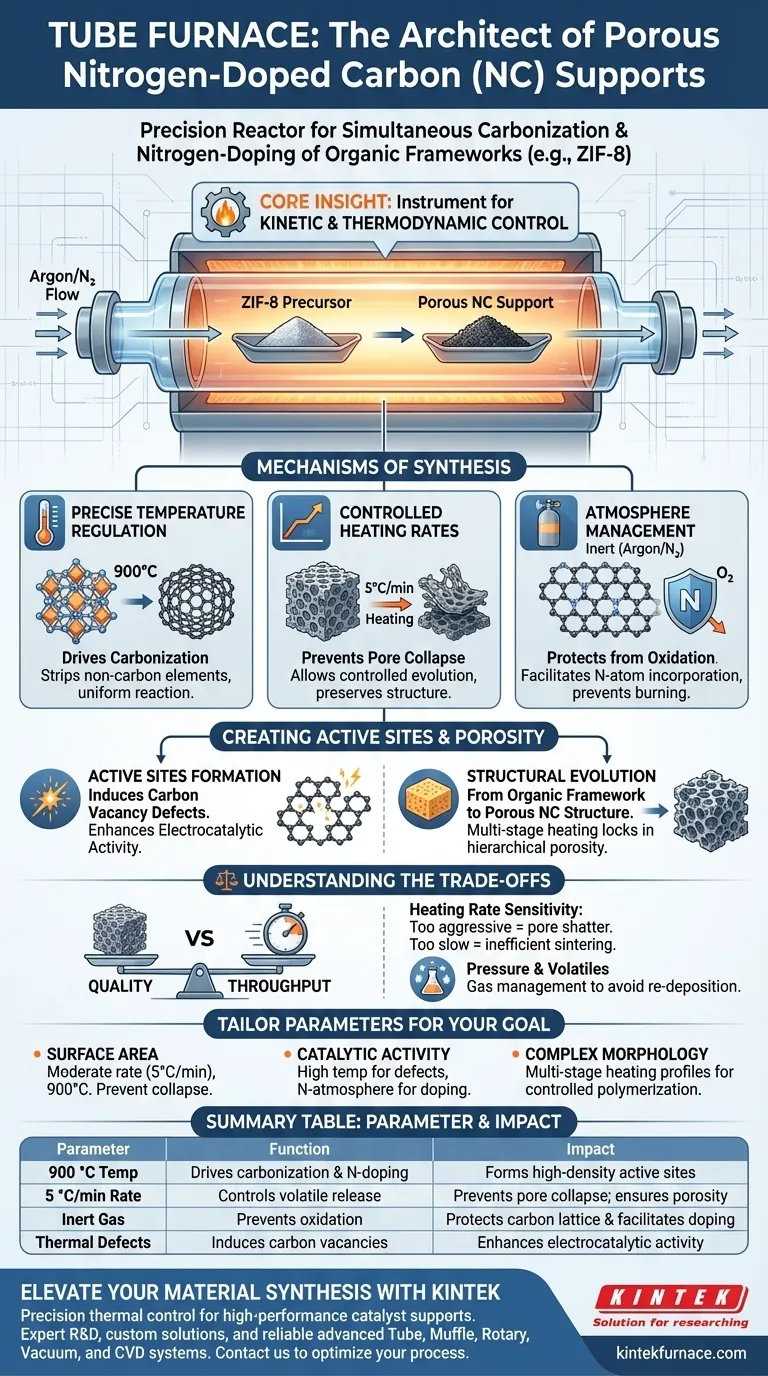
The tube furnace serves as a precision reactor that facilitates the simultaneous carbonization and nitrogen-doping of organic frameworks. By maintaining a stable 900 °C environment under an inert argon atmosphere, it transforms precursors like ZIF-8 into porous carbon supports characterized by high specific surface areas and a high density of active sites.
Core Insight A tube furnace is not merely a heating source; it is an instrument for kinetic and thermodynamic control. Its ability to regulate heating rates and maintain specific atmospheres ensures the organic framework decomposes systematically, preserving porosity while embedding nitrogen atoms into the carbon lattice.

Mechanisms of Synthesis
Precise Temperature Regulation
The primary function of the tube furnace is to provide a stable, high-temperature environment, typically targeting 900 °C for ZIF-8 precursors.
This thermal energy drives the carbonization process, stripping away non-carbon elements while reorganizing the remaining structure. The stability of the furnace ensures that the reaction proceeds uniformly throughout the material batch.
Controlled Heating Rates
Success depends on how the temperature is reached, not just the final set point. The tube furnace allows for a programmed ramp, such as 5 °C per minute.
A controlled heating rate is critical for maintaining structural integrity. Rapid heating can cause the sudden release of volatiles, leading to pore collapse. A steady, moderate rate allows the organic framework to evolve into a carbon structure without destroying the desired porosity.
Atmosphere Management
The tube furnace protects the sample from oxidation by maintaining a continuous flow of inert gas, such as argon or nitrogen.
This environment prevents the carbon from burning away (forming CO2) and instead facilitates the incorporation of nitrogen atoms into the carbon matrix. The inclusion of specific reducing gases can also be used to remove oxygen-containing groups, allowing for the fine-tuning of chemical properties without damaging the pore structure.
Creating Active Sites and Porosity
Formation of Active Sites
The high-temperature treatment does more than just carbonize; it activates the material. The process creates a high density of active sites suitable for subsequent metal atom loading.
Supplementary data suggests that this thermal treatment induces carbon vacancy defects. These defects are essential for enhancing the electrocatalytic activity of the final support, transforming the polymer network into a highly conductive system.
Structural Evolution
The furnace facilitates the transition from an organic framework to a porous nitrogen-doped carbon (NC) structure.
Advanced programming allows for multi-stage heating strategies. For example, an initial hold at lower temperatures can form intermediates, followed by a rise to higher temperatures to lock in hierarchical porosity. This ensures the final material has the specific surface area required for high-performance applications.
Understanding the Trade-offs
Heating Rate Sensitivity
While a 5 °C/min rate protects structure, it is a trade-off between quality and throughput.
If the heating rate is too aggressive, the rapid evolution of gases can shatter the delicate porous architecture. Conversely, extremely slow rates may be inefficient and allow for unwanted sintering of the material, potentially reducing the available surface area.
Pressure and Volatiles
Carbonization generates significant volatile byproducts. If these are not managed, they can alter the internal pressure of the tube or re-deposit on the sample.
In some setups, a temperature gradient is used (keeping the ends of the tube cooler) to condense these volatiles away from the reaction zone. Failure to manage internal pressure can lead to inconsistent doping levels or safety hazards.
Making the Right Choice for Your Goal
To optimize your nitrogen-doped carbon synthesis, tailor the furnace parameters to your specific objectives:
- If your primary focus is Surface Area: Adhere strictly to a moderate heating rate (e.g., 5 °C/min) and high carbonization temperature (900 °C) to prevent pore collapse while fully carbonizing the ZIF-8 framework.
- If your primary focus is Catalytic Activity: Ensure the temperature is sufficient to induce carbon vacancy defects, and consider using a nitrogen atmosphere to further facilitate doping.
- If your primary focus is Complex Morphology: Utilize the programmable features to create a multi-stage heating profile (e.g., dwell at intermediate temps) to control the polymerization of precursors before final carbonization.
Ultimately, the tube furnace acts as the architect of the material, where precise thermal control dictates the difference between a collapsed powder and a high-performance catalyst support.
Summary Table:
| Parameter | Function in NC Synthesis | Impact on Material |
|---|---|---|
| 900 °C Temperature | Drives carbonization & N-doping | Forms high-density active sites |
| 5 °C/min Ramp Rate | Controls volatile release | Prevents pore collapse; ensures porosity |
| Inert Atmosphere | Prevents oxidation (Argon/N2) | Protects carbon lattice & facilitates doping |
| Thermal Defects | Induces carbon vacancies | Enhances electrocatalytic activity |
Elevate Your Material Synthesis with KINTEK
Precision is the difference between a collapsed framework and a world-class catalyst support. Backed by expert R&D and manufacturing, KINTEK offers advanced Tube, Muffle, Rotary, Vacuum, and CVD systems designed for the rigorous demands of nitrogen-doped carbon synthesis. Our furnaces provide the stable thermal environments and programmable heating rates essential for preserving hierarchical porosity and optimizing active sites.
Whether you need a standard setup or a fully customizable solution for unique high-temperature research, KINTEK delivers the reliability your lab requires.
Ready to optimize your carbonization process? Contact us today to discuss your specific lab needs with our specialists.
Visual Guide
References
- Wensheng Jiao, Yunhu Han. All-round enhancement induced by oxophilic single Ru and W atoms for alkaline hydrogen oxidation of tiny Pt nanoparticles. DOI: 10.1038/s41467-025-56240-y
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- Vertical Laboratory Quartz Tube Furnace Tubular Furnace
People Also Ask
- What role does a laboratory tube furnace perform during the carbonization of LCNSs? Achieve 83.8% Efficiency
- What recent improvements have been made to lab tube furnaces? Unlock Precision, Automation & Safety
- What safety and reliability features are incorporated into a vertical tube furnace? Ensuring Safe, Consistent High-Temp Processing
- What are the key operational considerations when using a lab tube furnace? Master Temperature, Atmosphere & Safety
- How is a high-temperature tube furnace utilized in the synthesis of MoO2/MWCNTs nanocomposites? Precision Guide



















