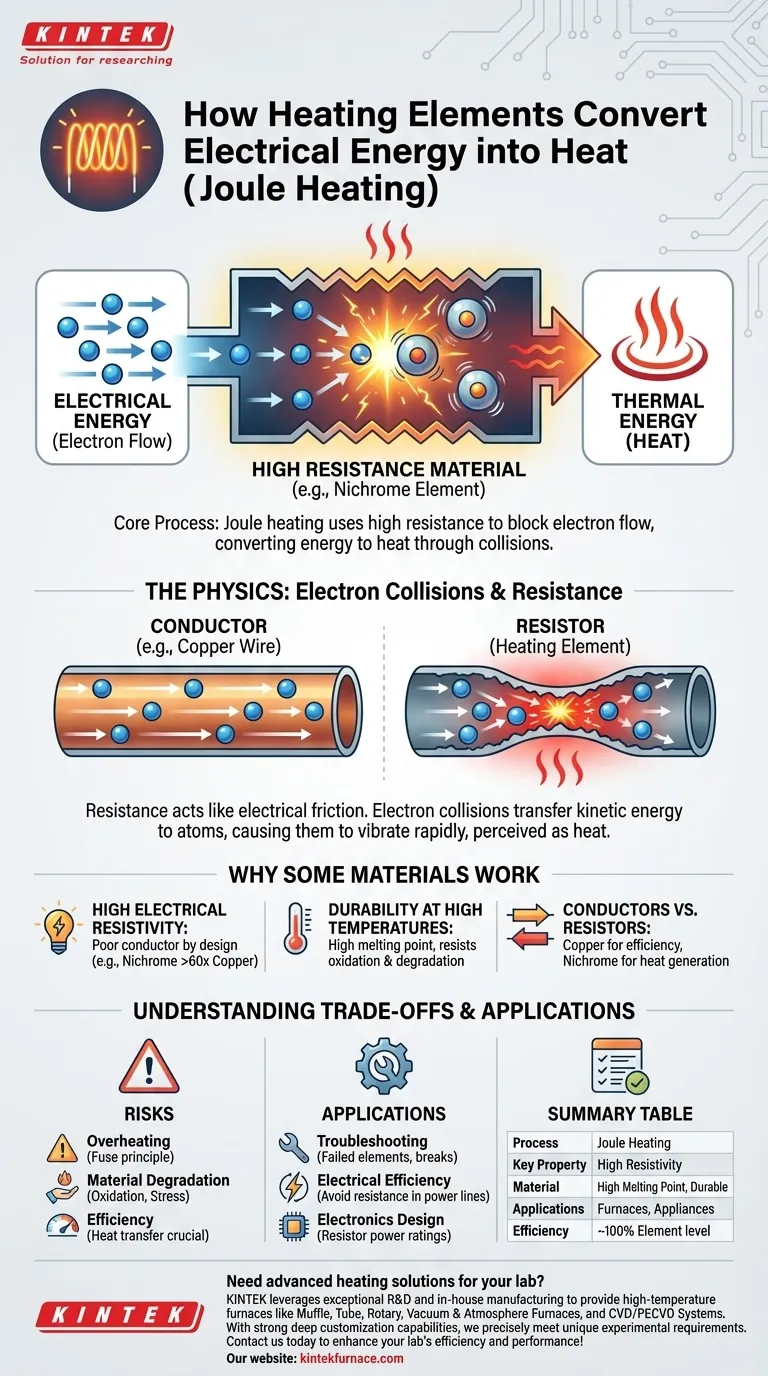
At its core, a heating element converts electrical energy into heat by deliberately resisting the flow of electricity. This process is known as Joule heating or resistive heating. When an electric current is forced through a material with high electrical resistance, the opposition to the flow causes the electrical energy to transform directly into thermal energy, making the element hot.
The conversion of electricity to heat is not a complex chemical reaction, but a fundamental physical process. It harnesses the microscopic friction created when electrons are forced to move through a material that actively resists their flow.
The Physics of Electrical Resistance
To understand how a heating element works, you must first understand the relationship between electricity, resistance, and heat.
What is Electric Current?
An electric current is simply the flow of electrons through a material. Think of it like water flowing through a pipe. A material that allows electrons to flow easily is called a conductor.
Introducing Resistance
Electrical resistance is a measure of a material's opposition to the flow of electric current. In our water pipe analogy, resistance is like a narrow, constricted section of the pipe that makes it harder for water to pass through.
The Microscopic View: Electron Collisions
At the atomic level, resistance occurs because the flowing electrons collide with the atoms of the material. As electrons are pushed through a resistive material, they bump into its atomic structure.
From Collision to Heat
Each of these collisions transfers kinetic energy from the moving electron to the atom. This transfer of energy causes the atoms in the material to vibrate more rapidly. This increased atomic vibration is what we perceive as heat.
Why Some Materials Are Used as Heating Elements
Not all materials are suitable for creating heat. The properties of a good heating element are specific and intentional.
High Electrical Resistivity
The most important property is high electrical resistivity. Unlike a copper wire, which is designed to conduct electricity with minimal energy loss, a heating element is made from a material that is a poor conductor by design.
Common materials include Nichrome (a nickel-chromium alloy), which has a resistivity over 60 times higher than copper. This high resistance is what enables the efficient conversion of electrical energy to heat.
Durability at High Temperatures
A material must not only get hot but also survive extreme temperatures without melting or degrading. Heating elements must have a high melting point and strong resistance to oxidation (rusting), which accelerates at high temperatures.
Conductors vs. Resistors
A household electrical system is a perfect example of these principles in action. The copper wiring in your walls has very low resistance to deliver power efficiently. The Nichrome wire in your toaster has very high resistance to turn that same power into heat.
Understanding the Trade-offs
The process of Joule heating is straightforward, but its application involves important design considerations and limitations.
The Risk of Overheating
The amount of heat generated is directly proportional to the resistance and the square of the current. If too much current flows or the heat is not allowed to dissipate, the element can quickly reach its melting point and fail. This is the same principle that allows a fuse to work—it's a wire designed to melt at a specific current.
Material Degradation Over Time
Even with oxidation-resistant alloys, heating elements degrade over their lifespan. The constant cycle of extreme heating and cooling causes stress and gradual oxidation, eventually making the element brittle and causing it to break. This is why heating elements in appliances like ovens and water heaters are common points of failure.
Energy Conversion vs. System Efficiency
The conversion of electrical energy into heat at the element itself is nearly 100% efficient. However, the overall efficiency of an appliance depends on how well that heat is transferred to its target—be it the water in a kettle, the air in a room, or the food in an oven. Poor insulation or design can waste much of the heat that is generated.
How to Apply This Principle
Understanding Joule heating is key to understanding the function and failure of countless electrical devices.
- If your primary focus is troubleshooting an appliance: A failed heating element is almost always a physical break in the resistive wire, which you can often test for with a simple continuity check.
- If your primary focus is electrical efficiency: This principle explains why low-resistance materials like copper are critical for power lines, as any resistance in the wire is simply wasting energy as heat.
- If your primary focus is electronics design: You now understand why resistors in a circuit get warm and have a power (wattage) rating—they are rated for how much heat they can safely dissipate.
By viewing resistance as a controlled form of electrical friction, you can demystify the operation of everything from a simple toaster to a complex industrial furnace.
Summary Table:
| Aspect | Key Details |
|---|---|
| Process | Joule heating converts electrical energy to heat through resistance to electron flow. |
| Key Property | High electrical resistivity (e.g., Nichrome alloy) for efficient heat generation. |
| Material Requirements | High melting point, oxidation resistance, durability under thermal cycling. |
| Applications | Used in appliances, industrial furnaces, and lab equipment for precise heating. |
| Efficiency | Nearly 100% at element level; overall depends on heat transfer and insulation. |
Need advanced heating solutions for your lab? KINTEK leverages exceptional R&D and in-house manufacturing to provide high-temperature furnaces like Muffle, Tube, Rotary, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems. With strong deep customization capabilities, we precisely meet unique experimental requirements. Contact us today to enhance your lab's efficiency and performance!
Visual Guide
Related Products
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- Multi Zone Laboratory Quartz Tube Furnace Tubular Furnace
People Also Ask
- How does a stainless steel reactor function within a muffle furnace for PET to graphene? Master Carbon Synthesis
- Why is a muffle furnace used to determine the ash content of biochar? Master Your Material Purity Analysis
- What role does a muffle furnace play in g-C3N4 synthesis? Mastering Thermal Polycondensation for Semiconductors
- What is the primary role of a muffle furnace in the annealing process of AlCrTiVNbx alloys? Enhance Alloy Strength
- What role does a muffle furnace play in the conversion of S-1@TiO2? Achieve Precision Calcination of Nanospheres



















