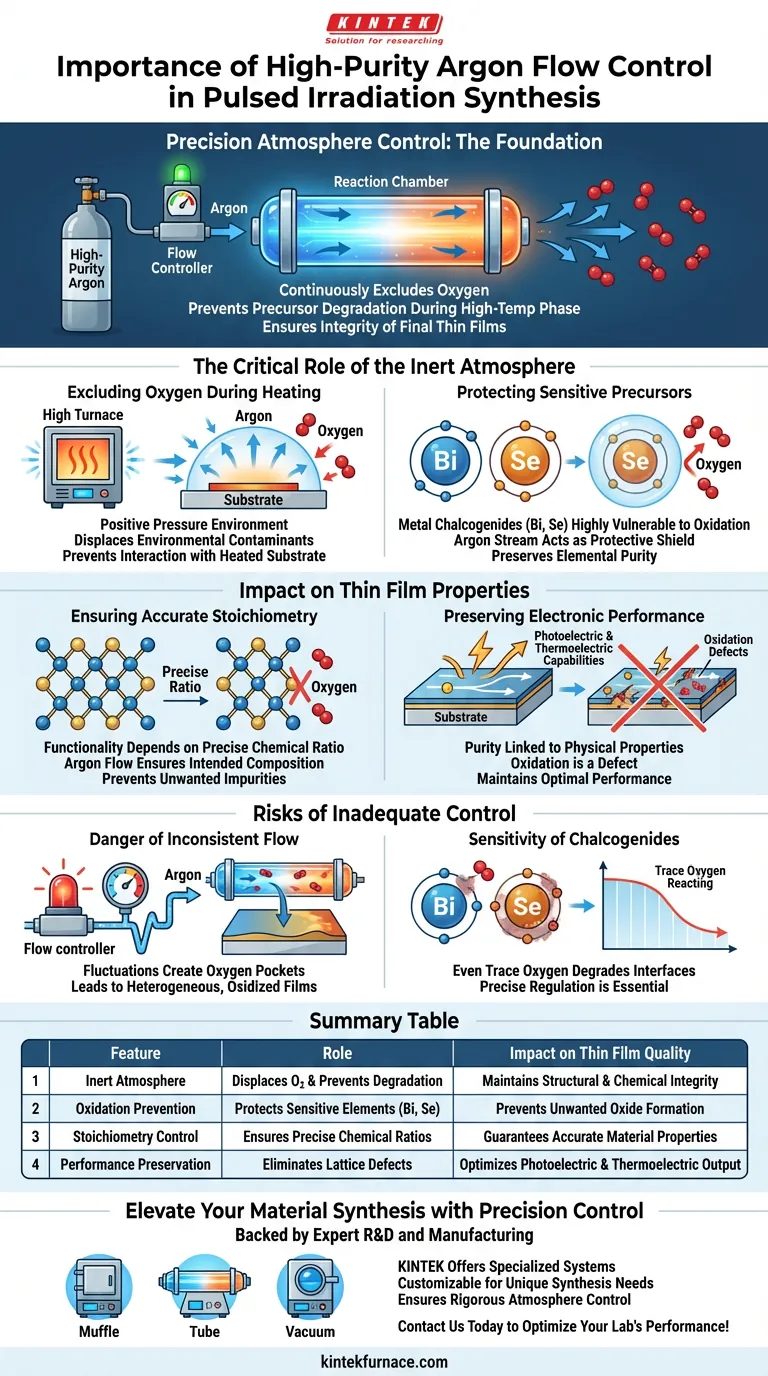
Precision atmosphere control is the foundation of successful Pulsed Irradiation Synthesis. High-purity Argon flow control equipment is strictly necessary to establish and maintain an inert environment within the reaction chamber. By continuously excluding oxygen, this equipment prevents the degradation of sensitive precursors during the high-temperature reaction phase, ensuring the structural and chemical integrity of the final thin films.
The primary function of Argon flow control is to prevent the oxidation of metal chalcogenides like bismuth and selenium. Without this inert barrier during high-temperature processing, the chemical composition shifts, severely compromising the photoelectric and thermoelectric performance of the synthesized thin films.

The Critical Role of the Inert Atmosphere
Excluding Oxygen During Heating
Pulsed irradiation involves distinct high-temperature phases designed to trigger chemical synthesis.
In this heated state, materials are exponentially more reactive to environmental contaminants. The Argon flow control equipment creates a positive pressure environment that physically displaces oxygen, preventing it from interacting with the heated substrate.
Protecting Sensitive Precursors
Certain materials used in this process, specifically metal chalcogenides such as bismuth (Bi) and selenium (Se), are highly vulnerable to oxidation.
If exposed to oxygen while heated, these elements will form oxides rather than the intended compound. The Argon stream acts as a protective shield, preserving the elemental purity required for the reaction.
Impact on Thin Film Properties
Ensuring Accurate Stoichiometry
The functionality of a thin film depends heavily on its stoichiometry—the precise ratio of its chemical components.
Oxygen contamination alters this ratio, introducing unwanted impurities into the lattice structure. High-purity Argon flow ensures that the final chemical composition matches the intended design without deviation.
Preserving Electronic Performance
The physical properties of the film, particularly its photoelectric and thermoelectric capabilities, are directly linked to its purity.
Oxidation acts as a defect within the material, impeding electron flow and energy conversion. By maintaining a strictly inert atmosphere, the equipment preserves the optimal performance characteristics of the synthesized film.
Risks of Inadequate Control
The Danger of Inconsistent Flow
Using high-purity Argon is insufficient if the flow control equipment cannot maintain a stable environment.
Fluctuations in flow can create temporary pockets where oxygen may re-enter the chamber or fail to be flushed out completely. This inconsistency leads to heterogeneous films where parts of the sample are oxidized and unusable.
The Sensitivity of Chalcogenides
Materials like bismuth and selenium do not tolerate "mostly" inert environments well.
Even trace amounts of oxygen reacting during the thermal pulse can degrade the sharp interfaces required for high-performance thin films. The equipment must offer precise regulation to ensure total exclusion throughout the entire synthesis window.
Ensuring Synthesis Success
To maximize the quality of your thin films, align your equipment strategy with your specific material goals.
- If your primary focus is chemical precision: Prioritize flow control systems with high leak integrity to ensure the absolute exclusion of oxygen, protecting the stoichiometry of bismuth and selenium.
- If your primary focus is device efficiency: Maintain a constant, uninterrupted flow of high-purity Argon to prevent micro-oxidation defects that degrade photoelectric and thermoelectric performance.
Rigorous management of the reaction atmosphere is the invisible prerequisite for high-performance thin film fabrication.
Summary Table:
| Feature | Role in Pulsed Irradiation Synthesis | Impact on Thin Film Quality |
|---|---|---|
| Inert Atmosphere | Displaces oxygen and prevents precursor degradation | Maintains structural and chemical integrity |
| Oxidation Prevention | Protects sensitive elements like Bismuth (Bi) and Selenium (Se) | Prevents unwanted oxide formation |
| Stoichiometry Control | Ensures precise chemical ratios during reaction | Guarantees accurate material properties |
| Performance Preservation | Eliminates lattice defects caused by contaminants | Optimizes photoelectric and thermoelectric output |
Elevate Your Material Synthesis with Precision Control
Don't let oxygen contamination compromise your research. Backed by expert R&D and manufacturing, KINTEK offers specialized high-temperature furnace systems, including Muffle, Tube, and Vacuum configurations, all customizable for your unique synthesis needs.
Our equipment ensures the rigorous atmosphere control required for sensitive Pulsed Irradiation Synthesis, protecting your thin films from the first thermal pulse to the final cooling phase. Contact us today to optimize your lab's performance!
Visual Guide
References
- Yuxuan Zhang, Johnny C. Ho. Pulse irradiation synthesis of metal chalcogenides on flexible substrates for enhanced photothermoelectric performance. DOI: 10.1038/s41467-024-44970-4
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- Magnesium Extraction and Purification Condensing Tube Furnace
- 304 316 Stainless Steel High Vacuum Ball Stop Valve for Vacuum Systems
- Ultra Vacuum Electrode Feedthrough Connector Flange Power Lead for High Precision Applications
- CF KF Flange Vacuum Electrode Feedthrough Lead Sealing Assembly for Vacuum Systems
People Also Ask
- How does a Mass Flow Controller (MFC) regulate TCNF morphology? Achieve Precise Carbon Nanofiber Growth
- What are the functions of a tungsten wire basket and a quartz crucible? Enhancing Purity in Vacuum Evaporation
- Why must alloy samples be sealed in vacuum-evacuated fused silica containers during diffusion annealing processes?
- Why is a benchtop forced air drying oven preferred for microalgae-based nanomaterials? Enhance Powder Quality
- What is the function of the laboratory-scale condensation collection device? Optimize Multi-Stage Magnesium Separation
- Why is zirconia grinding media preferred for NN-10ST ceramic powders? Ensure Purity & Dielectric Performance
- What customization options are available for laboratory furnaces? Tailor Your Furnace for Precise Thermal Control
- Why use a graphite box for WS2 sulfurization? Essential for High-Quality Thin Film Synthesis



















