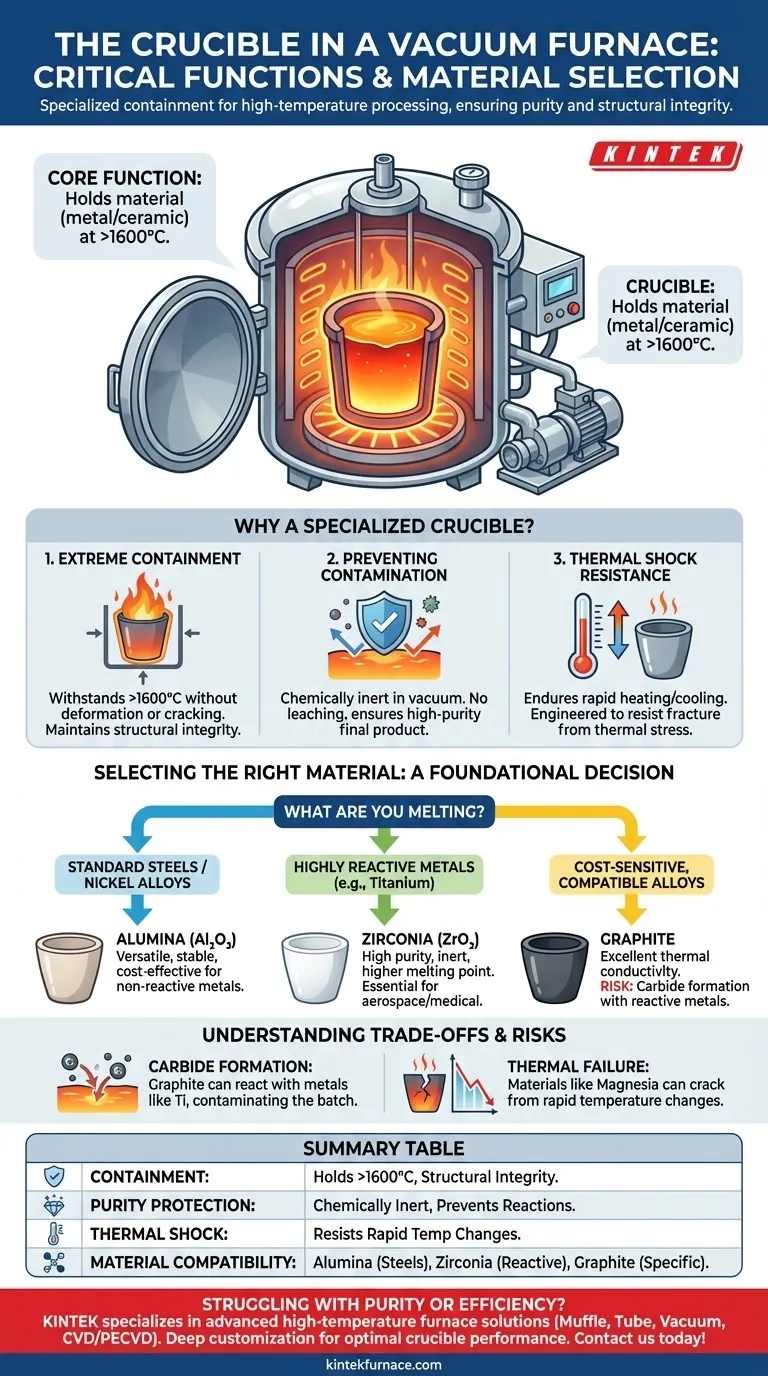
At its core, the crucible is the specialized container designed to hold material, typically metal or ceramic, during high-temperature melting and processing inside a vacuum furnace. It is engineered to withstand extreme heat and thermal stress while remaining chemically stable, ensuring the purity of the material it contains. The crucible may also be integrated with systems for automatic pouring or material handling.
The function of a crucible extends far beyond simple containment. It is an active and critical component whose material composition directly dictates the success of the process, preventing catastrophic reactions and ensuring the final product meets its required purity and quality specifications.

Why a Vacuum Furnace Needs a Specialized Crucible
A vacuum furnace creates a controlled, low-pressure environment to prevent oxidation and contamination during the heating of materials. The crucible is the component that directly interfaces with the hot material within this pristine environment, making its role absolutely critical.
Containing the Charge Under Extreme Conditions
The most basic function of the crucible is to securely hold the solid or molten metal, known as the "charge." It must maintain its structural integrity at exceptionally high temperatures, often exceeding 1600°C (2900°F), without deforming, cracking, or failing.
Preventing Chemical Contamination
The primary advantage of a vacuum furnace is producing high-purity materials. The crucible must be chemically inert, meaning it cannot react with the molten metal it holds. An incompatible crucible will leach impurities into the melt, defeating the entire purpose of using a vacuum process.
Withstanding Severe Thermal Shock
A crucible endures rapid temperature changes as the furnace heats up and cools down. It must be engineered to resist thermal shock—the stress induced by sudden temperature gradients—which could otherwise cause it to fracture catastrophically, spilling molten metal and severely damaging the furnace.
Selecting the Right Crucible Material
The choice of crucible material is not arbitrary; it is a critical engineering decision based on the material being processed and the target temperature. An incorrect choice will lead to process failure.
Matching Material to the Metal
The guiding principle is chemical compatibility. The crucible's composition must be stable and non-reactive with the specific alloy being melted at the intended process temperature.
Common Material: Alumina (Al₂O₃)
Alumina is a versatile and widely used ceramic for crucibles. It is the go-to choice for melting many steels, nickel-based superalloys, and other relatively non-reactive metals due to its high-temperature stability and reasonable cost.
High-Purity Choice: Zirconia (ZrO₂)
For more demanding applications involving highly reactive metals (like titanium) or higher process temperatures, zirconia is often required. It is more inert and has a higher melting point than alumina, providing the purity needed for aerospace and medical-grade alloys.
Special Application: Graphite
Graphite crucibles are used for melting certain metals and alloys. They offer excellent thermal conductivity and are cost-effective. However, their use is limited by a significant risk.
Understanding the Trade-offs and Risks
Choosing a crucible involves balancing performance, cost, and risk. Overlooking the potential downsides of a material choice can lead to costly failures.
The Risk of Carbide Formation
The most significant risk with graphite crucibles is their tendency to react with certain metals to form carbides. If a graphite crucible is used to melt titanium, for example, carbon will dissolve into the melt, forming titanium carbide and contaminating the entire batch.
The Danger of Thermal Failure
Some materials, like magnesia, offer high-temperature stability but are extremely susceptible to thermal shock. If not heated and cooled on a very slow, controlled schedule, they can easily crack, leading to a complete loss of the melt.
The Impact of Incorrect Selection
Using the wrong crucible doesn't just risk one batch. It can lead to furnace downtime, costly repairs, wasted energy, and a final product that does not meet specifications, rendering the entire operation a failure.
How to Choose the Right Crucible for Your Process
Your choice must be driven by the specific requirements of your material and process goal.
- If your primary focus is melting standard steels or nickel alloys: An Alumina (Al₂O₃) crucible is typically the most reliable and cost-effective starting point.
- If your primary focus is working with highly reactive metals like titanium or refractory alloys: You must use a high-purity ceramic like Zirconia (ZrO₂) to prevent melt contamination.
- If your primary focus is cost-sensitive melting of specific, compatible alloys: Graphite may be a viable option, but only after you have confirmed it will not form unwanted carbides with your specific metal.
Making the right crucible choice is a foundational decision that protects your material purity, your equipment, and the ultimate success of your vacuum furnace operation.
Summary Table:
| Function | Key Details |
|---|---|
| Containment | Holds solid or molten materials at temperatures over 1600°C, ensuring structural integrity. |
| Purity Protection | Chemically inert to prevent reactions and contamination in vacuum environments. |
| Thermal Shock Resistance | Engineered to withstand rapid temperature changes without cracking or failure. |
| Material Compatibility | Choice depends on metal type (e.g., Alumina for steels, Zirconia for reactive metals). |
Struggling with material purity or furnace efficiency in your lab? KINTEK specializes in advanced high-temperature furnace solutions, including Muffle, Tube, Rotary, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems. With our strong R&D and in-house manufacturing, we offer deep customization to precisely match your experimental needs—ensuring optimal crucible performance and process success. Contact us today to discuss how we can enhance your operations with tailored solutions!
Visual Guide
Related Products
- Multi Zone Laboratory Quartz Tube Furnace Tubular Furnace
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 9MPa Air Pressure Vacuum Heat Treat and Sintering Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
People Also Ask
- What steps are involved in the installation of a multi zone tube furnace? Ensure Precision and Safety for Your Lab
- What advantages do multi zone tube furnaces offer for chemical reaction studies? Achieve Precise Thermal Control
- How does a multi-zone tube furnace achieve precise temperature gradient control? Master MoS2 Isotope Monolayer Synthesis
- What preparations are needed before starting a multi zone tube furnace? Ensure Safety and Accuracy in Your Lab
- What are the benefits of integrating multiple heating zones in a tube furnace? Unlock Precise Thermal Control



















