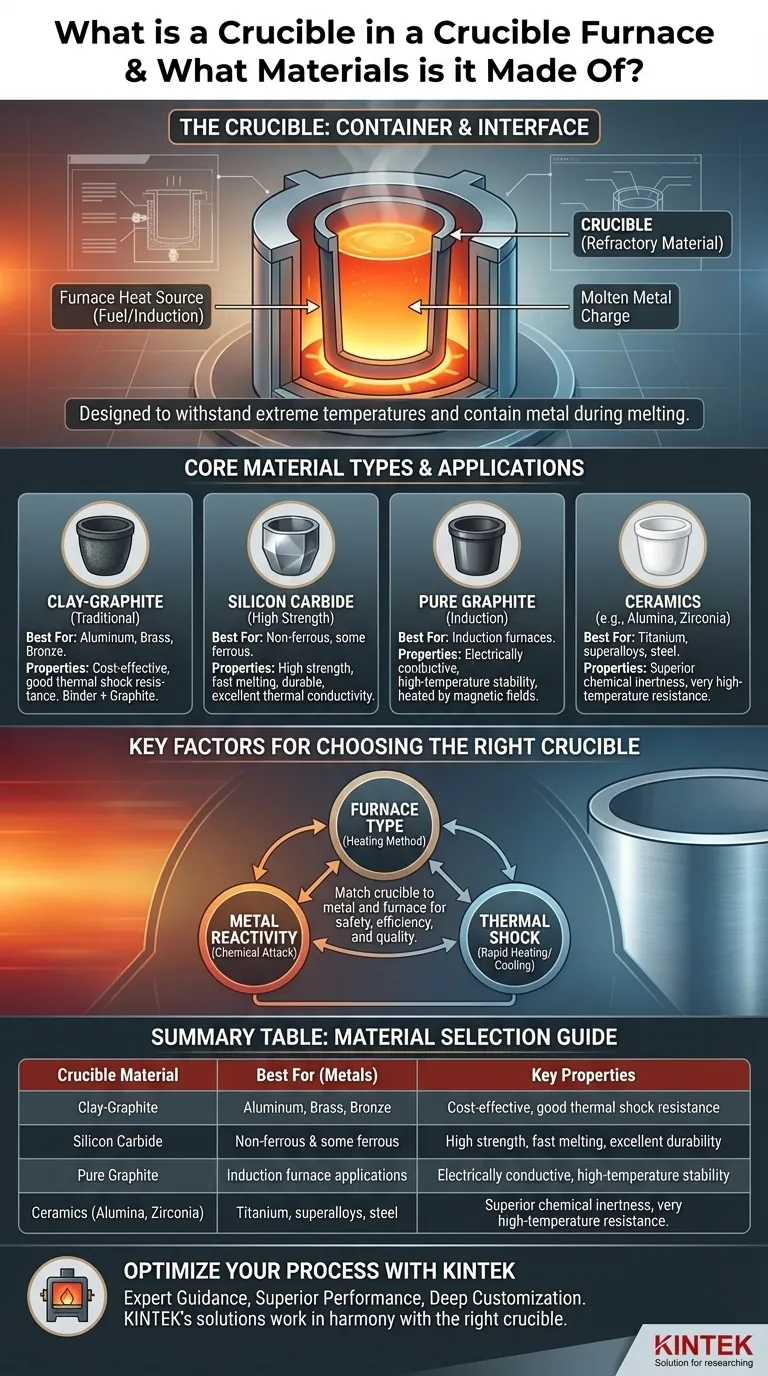
In a crucible furnace, the container is called a crucible. It is designed to withstand extreme temperatures and is typically manufactured from refractory materials like graphite, clay-graphite mixtures, silicon carbide, or high-purity ceramics. The specific material choice is dictated by the metal being melted and the type of furnace being used.
While the simple answer is "a crucible made of heat-resistant material," the critical insight is that the choice of material is not universal. It involves a precise match between the crucible's properties, the chemical reactivity of the metal you're melting, and the heating method of your furnace.

The Role and Function of the Crucible
A crucible serves one primary purpose: to securely contain metal as it is heated to its melting point and beyond. It acts as the direct interface between the heat source and the metal charge.
The Foundation: Refractory Materials
The term refractory simply means a material is physically and chemically stable at high temperatures. This is the single most important characteristic of any crucible.
Without this property, the container would melt, crack, or chemically react with the molten metal, leading to catastrophic failure and contamination of the final product.
Core Material Types and Their Applications
The material used to construct a crucible is chosen based on melting temperature, resistance to chemical reaction, and thermal shock resistance.
- Clay-Graphite: This is a traditional and common material. The clay acts as a binder, while the graphite provides excellent thermal conductivity and resistance to thermal shock. These are workhorses for non-ferrous metals like aluminum, brass, and bronze.
- Silicon Carbide: These crucibles offer superior physical strength and even better thermal conductivity than clay-graphite. This allows for faster melting cycles and makes them highly durable, suitable for both non-ferrous and some ferrous applications.
- Pure Graphite: Used in specialized applications, high-purity graphite is often required for induction furnaces. Its conductive properties allow it to be heated directly by the furnace's magnetic fields.
- Ceramics (e.g., Alumina, Zirconia): For extremely high-temperature applications or when melting highly reactive metals (like titanium or superalloys), advanced ceramics are used. They provide superior chemical inertness, preventing contamination of the melt.
Understanding the Trade-offs: Matching Crucible to Metal
Choosing the wrong crucible is a common and costly mistake. The interaction between the molten metal and the crucible material is a critical factor.
The Challenge of Chemical Reactivity
Molten metals are highly reactive. A crucible that works perfectly for aluminum may be aggressively attacked by molten iron.
For example, using a simple clay crucible for steel melting would likely fail, as the temperatures are too high and the molten iron is chemically aggressive.
The Impact of the Furnace Type
The heating method also dictates crucible choice.
- Fuel-Fired Furnaces: In a gas or oil furnace, the crucible is heated by external flames. Here, high thermal conductivity is key to efficiently transfer heat to the metal inside. Silicon carbide and clay-graphite are excellent choices.
- Induction Furnaces: These furnaces use electromagnetic fields to heat the metal (or the crucible itself). A conductive crucible like graphite is often used because the furnace can heat it directly, which in turn melts the metal. In other cases, a non-conductive ceramic crucible is used, and the magnetic field passes through it to heat the metal charge directly.
The Problem of Thermal Shock
Rapid heating or cooling can cause a crucible to crack—an event known as thermal shock. Materials like graphite and silicon carbide have excellent resistance to this, while some ceramics can be more sensitive and require carefully controlled heating cycles.
Making the Right Choice for Your Goal
Your selection must align with your specific metallurgical process to ensure safety, efficiency, and final product quality.
- If your primary focus is melting non-ferrous metals like aluminum or brass in a fuel-fired furnace: A clay-graphite or silicon carbide crucible is your most reliable and cost-effective choice.
- If your primary focus is melting steel or high-temperature alloys: You must use a specialized high-purity ceramic or a crucible specifically rated for ferrous metals.
- If your primary focus is using an induction furnace: Your choice depends on the furnace design; select either a conductive graphite-based crucible or a non-conductive ceramic one as specified by the manufacturer.
- If your primary focus is melting highly reactive metals like titanium: An inert, hermetically sealed ceramic crucible is non-negotiable to prevent atmospheric contamination.
Ultimately, the crucible is not just a container; it is an active and critical component in the melting process.
Summary Table:
| Crucible Material | Best For (Metals) | Key Properties |
|---|---|---|
| Clay-Graphite | Aluminum, Brass, Bronze | Cost-effective, good thermal shock resistance |
| Silicon Carbide | Non-ferrous & some ferrous | High strength, fast melting, excellent durability |
| Pure Graphite | Induction furnace applications | Electrically conductive, high-temperature stability |
| Ceramics (Alumina, Zirconia) | Titanium, superalloys, steel | Superior chemical inertness, very high-temperature resistance |
Optimize Your Metal Melting Process with KINTEK
Choosing the right crucible is critical for the safety, efficiency, and quality of your lab work. The wrong choice can lead to contamination, crucible failure, and wasted resources.
KINTEK's advanced high-temperature furnace solutions are designed to work in perfect harmony with the right crucible for your application. We understand that your success depends on precise thermal processing.
We provide more than just equipment; we provide a solution tailored to your unique needs:
- Expert Guidance: Our team helps you select the ideal crucible and furnace combination for your specific metal and process requirements.
- Superior Performance: Our Muffle, Tube, and Vacuum & Atmosphere Furnaces offer the precise temperature control and uniform heating your experiments demand.
- Deep Customization: Leveraging our exceptional in-house R&D and manufacturing, we can customize furnace systems to integrate seamlessly with specialized crucibles for reactive metals or unique experimental setups.
Don't let crucible selection compromise your results. Let our experts help you build the perfect system for reliable, repeatable metal melting.
Contact KINTEK today to discuss your application and receive a personalized recommendation.
Visual Guide
Related Products
- Chairside Dental Porcelain Zirconia Sintering Furnace with Transformer for Ceramic Restorations
- Multi Zone Laboratory Quartz Tube Furnace Tubular Furnace
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
- 1400℃ Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
People Also Ask
- What are the recommended maintenance practices for dental furnaces? Ensure Precision and Longevity for Your Lab
- What is the importance of dental furnaces in dentistry? Ensure Strong, Precise Dental Restorations
- What role does temperature range and accuracy play in dental furnace performance? Ensure Precision for Superior Dental Restorations
- What are the primary functions of ceramic dental furnaces? Achieve Precision and Durability in Dental Restorations
- What are the benefits of using dental sintering and porcelain furnaces? Enhance Strength, Aesthetics, and Efficiency















