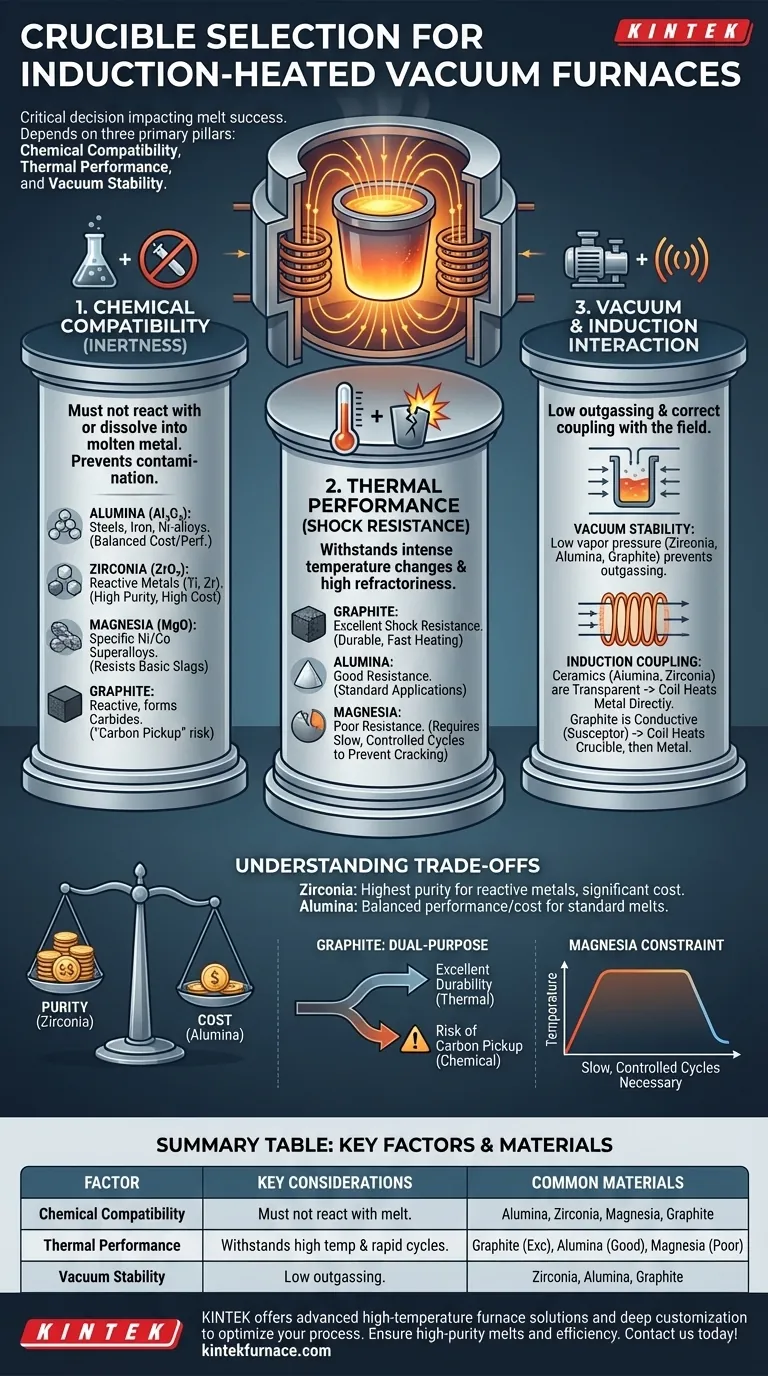
Selecting the right crucible for an induction-heated vacuum furnace is a critical decision that directly impacts the success of your melt. The choice depends on three primary factors: chemical compatibility with the metal being melted, the material's ability to withstand extreme process temperatures, and its stability under vacuum. Your main options are refractory ceramics like alumina, zirconia, and magnesia, or a conductive material like graphite, each suited for specific applications.
A crucible is not merely a container; it is an active component in a high-energy metallurgical system. The optimal choice is a material that remains chemically inert to your alloy at peak temperature, resists thermal shock from rapid heating cycles, and does not outgas under vacuum, thereby ensuring melt purity and furnace integrity.

The Three Pillars of Crucible Selection
Choosing a crucible requires a systematic evaluation of its interaction with the metal, the heat, and the furnace environment. Neglecting any one of these pillars can lead to contamination, crucible failure, and costly downtime.
Pillar 1: Chemical Compatibility
This is the most critical factor. The crucible material must not react with or dissolve into the molten metal, as this would contaminate the final product.
- Alumina (Al₂O₃): This is the workhorse material for many applications. It is relatively inexpensive and demonstrates good stability when melting iron, steels, and many nickel-based alloys.
- Zirconia (ZrO₂): This is the material of choice for high-temperature applications involving highly reactive metals, such as titanium, zirconium, and other refractory alloys. Its superior chemical inertness prevents oxygen contamination in these sensitive melts.
- Magnesia (MgO): Used for melting specific nickel and cobalt-based superalloys where alumina or zirconia are not suitable. It offers excellent resistance to basic slags.
- Graphite: While it has excellent thermal properties, graphite is reactive. It will readily dissolve into certain molten metals (like iron or titanium), forming carbides. This "carbon pickup" can be a desirable effect in some processes but is a major source of contamination in others.
Pillar 2: Thermal Performance
The crucible must endure the intense and rapid temperature changes inherent to induction heating.
- Refractoriness: This is a material's ability to withstand high temperatures without degrading or melting. The crucible's melting point must be significantly higher than the maximum processing temperature of your alloy.
- Thermal Shock Resistance: Induction heating is extremely fast, creating immense thermal stress as the crucible expands. The subsequent cooling cycle creates stress again. Materials with poor thermal shock resistance, like magnesia, are prone to cracking if not heated and cooled on a very slow, controlled schedule.
Pillar 3: Interaction with the Furnace Environment
The crucible must perform correctly within the unique conditions of an induction-heated vacuum chamber.
- Vacuum Stability: At high temperatures and low pressures, some materials can "outgas," releasing volatile elements. A suitable crucible must have low vapor pressure to avoid contaminating the vacuum environment and the melt itself.
- Induction Coupling: Ceramic crucibles (alumina, zirconia) are transparent to the electromagnetic field, meaning the induction coil heats the conductive metal charge directly. In contrast, a graphite crucible is electrically conductive and is heated directly by the field, which in turn heats the metal charge via conduction and radiation. This makes graphite a "susceptor," which can be useful for melting non-conductive materials or improving thermal uniformity.
Understanding the Trade-offs
There is no single "best" crucible. Your selection will always be a balance of performance, cost, and operational constraints.
Cost vs. Purity
Zirconia offers the highest level of purity for reactive metals but comes at a significant cost premium over alumina. For melting standard steels where minor alumina inclusions are tolerable, using an expensive zirconia crucible is unnecessary.
Graphite: A Dual-Purpose Material
Graphite's high thermal conductivity and excellent thermal shock resistance make it very durable. However, the risk of carbide formation makes it completely unsuitable for producing low-carbon alloys. You must decide if potential carbon pickup is an acceptable risk, an unwanted contaminant, or a desired feature for your specific process.
Operational Constraints
Materials like magnesia may have ideal chemical resistance for a particular superalloy, but their poor thermal shock resistance imposes a significant operational burden. You must commit to slow, carefully programmed heating and cooling ramps to prevent catastrophic crucible failure.
Making the Right Choice for Your Melt
Your application dictates the correct material. Use this guide to align your crucible choice with your primary goal.
- If your primary focus is melting standard steels, iron, or common nickel alloys: Alumina offers the best balance of performance and cost.
- If your primary focus is melting highly reactive metals like titanium or alloys at extreme temperatures: Zirconia is the required choice to prevent melt contamination and ensure purity.
- If your primary focus is melting alloys where carbon content must be minimized: Strictly avoid graphite crucibles to prevent unwanted carbon pickup from the crucible wall.
- If your primary focus is melting specific alloys where magnesia is specified: You must use magnesia, but be prepared to implement a slow, controlled heating and cooling profile to prevent cracking.
A methodical evaluation of these factors transforms crucible selection from a guess into a predictable component of a successful, high-purity melting process.
Summary Table:
| Factor | Key Considerations | Common Materials |
|---|---|---|
| Chemical Compatibility | Must not react with molten metal to avoid contamination | Alumina, Zirconia, Magnesia, Graphite |
| Thermal Performance | Withstands high temperatures and rapid heating cycles | Alumina (good), Graphite (excellent), Magnesia (poor) |
| Vacuum Stability | Low outgassing to maintain purity and furnace integrity | Zirconia, Alumina, Graphite |
Struggling with crucible selection for your induction-heated vacuum furnace? KINTEK leverages exceptional R&D and in-house manufacturing to provide advanced high-temperature furnace solutions tailored to your needs. Our product line includes Muffle, Tube, Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems, all backed by strong deep customization capabilities to precisely meet your unique experimental requirements. Ensure high-purity melts and operational efficiency—contact us today to discuss how we can optimize your process!
Visual Guide
Related Products
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- Laboratory Muffle Oven Furnace with Bottom Lifting
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
People Also Ask
- What is the function of high-vacuum encapsulated quartz tubes for Ce2(Fe, Co)17? Ensure Phase Purity and Stability
- What industrial and research applications are tube furnaces used for? Unlock Precise Thermal Processing Solutions
- What is the working principle of a vacuum tube furnace? Master Precise High-Temperature Processing
- What is the primary function of a vacuum-sealed quartz tube in MnBi2Te4 growth? Ensure High-Purity Crystal Synthesis
- How to clean a tube furnace? A Step-by-Step Guide to Safe and Effective Maintenance



















