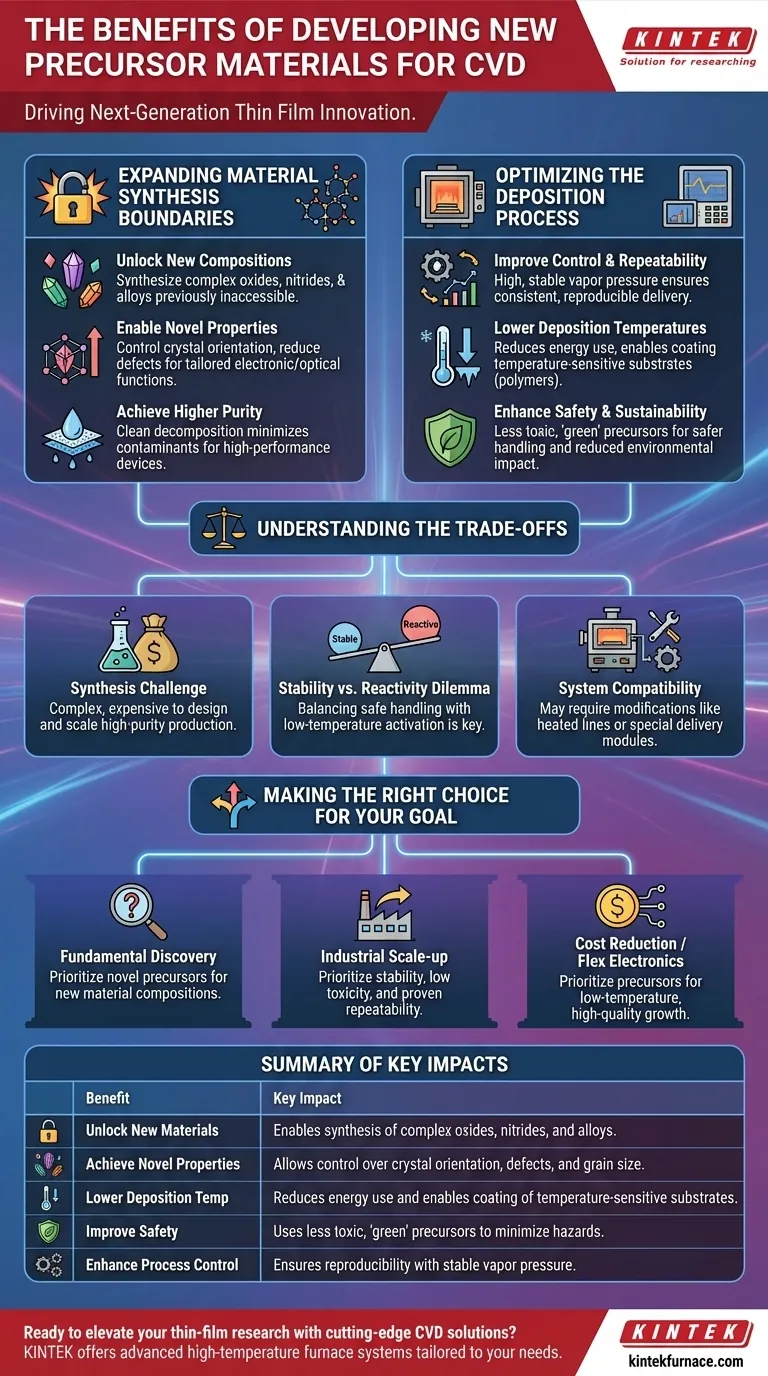
Developing new precursor materials for Chemical Vapor Deposition (CVD) is the primary driver for creating next-generation thin films. These new chemicals are not just minor improvements; they fundamentally expand the library of materials we can synthesize and refine the quality, safety, and efficiency of the entire deposition process itself. The core benefits are the ability to deposit previously inaccessible materials, achieve novel film properties, lower process temperatures, and improve safety and reproducibility.
The limitations of a CVD system are often not in the furnace hardware, but in the chemistry of the precursors it can use. Therefore, innovating precursor materials is the most critical path to overcoming existing barriers in advanced material synthesis.

Expanding the Boundaries of Material Synthesis
The most significant impact of new precursor development is its ability to push the boundaries of what materials can be created and what properties they can possess.
Unlocking New Material Compositions
Many technologically important materials, such as complex oxides, nitrides, or certain metal alloys, are difficult or impossible to deposit using traditional precursors. This is often because no stable, volatile chemical source exists for one of the required elements.
Developing new precursors directly addresses this gap, making it possible to synthesize entirely new classes of thin films that were previously inaccessible through CVD.
Enabling Novel Film Properties
The molecular structure of a precursor directly influences the final properties of the deposited film. A well-designed precursor can promote a specific crystal orientation, reduce defects, or control grain size.
This allows researchers to create films with novel electronic, optical, or mechanical functionalities, moving beyond simply depositing a material to engineering its performance at a molecular level.
Achieving Higher Purity
A superior precursor is designed to decompose cleanly at the substrate surface, leaving behind only the desired elements. This minimizes the incorporation of contaminants like carbon or halogens into the film.
This works in concert with high-purity system components, like quartz furnace tubes, to ensure the final product has the highest possible material purity, which is critical for high-performance electronic and optical devices.
Optimizing the Deposition Process Itself
Beyond creating new materials, new precursors can make the CVD process more efficient, controllable, and safer.
Improving Process Control and Repeatability
An ideal precursor has a high and stable vapor pressure, which allows for consistent, repeatable delivery into the reaction chamber. It should also be thermally stable enough to not decompose prematurely in the gas lines.
This stability is essential for leveraging the advanced control systems of modern CVD furnaces, ensuring that each deposition run is identical and that results are reproducible—a cornerstone of both scientific research and industrial manufacturing.
Lowering Deposition Temperatures
A major goal of precursor design is to lower the energy required for the chemical reaction to occur. Precursors that decompose at lower temperatures have significant advantages.
They reduce the thermal budget of the process, saving energy and minimizing stress on the substrate. This also enables deposition on temperature-sensitive materials, such as polymers or plastics, which would be damaged by traditional high-temperature CVD.
Enhancing Safety and Sustainability
Many conventional precursors are highly toxic, pyrophoric (ignite spontaneously in air), or environmentally hazardous. A key area of modern research is the development of "green" precursors.
These new materials are designed to be less toxic and safer to handle, store, and transport. This reduces operational risk, simplifies waste disposal, and makes the entire CVD process more sustainable.
Understanding the Trade-offs
While the benefits are significant, the development and use of new precursors involve inherent challenges and compromises.
The Synthesis Challenge
Designing and creating a novel precursor molecule with ideal properties is a complex and often expensive chemical synthesis task. Scaling this synthesis to produce high-purity material in large quantities can be a major barrier to adoption.
The Stability vs. Reactivity Dilemma
There is an intrinsic trade-off between a precursor's stability and its reactivity. A highly reactive molecule may enable low-temperature deposition but can be dangerously unstable and difficult to handle.
Conversely, an extremely stable precursor is very safe but may require impractically high temperatures to decompose, limiting its utility. Finding the right balance is a central challenge.
System Compatibility
A new precursor may have different physical properties that require modifications to the CVD system. It might require heated gas lines, a different type of delivery module, or specialized vacuum components. The entire system must be considered for compatibility before a new chemical can be implemented.
Making the Right Choice for Your Goal
The ideal precursor is always defined by the specific goal of your project.
- If your primary focus is fundamental materials discovery: Prioritize novel precursors that enable entirely new material compositions, even if they are more challenging to handle.
- If your primary focus is process industrialization and scale-up: Prioritize precursors that offer exceptional stability, low toxicity, high vapor pressure, and proven repeatability.
- If your primary focus is cost reduction or flexible electronics: Prioritize precursors that enable high-quality film growth at the lowest possible deposition temperature.
Ultimately, precursor innovation is the engine that drives the entire field of advanced thin-film technology forward.
Summary Table:
| Benefit | Key Impact |
|---|---|
| Unlock New Materials | Enables synthesis of complex oxides, nitrides, and alloys previously inaccessible. |
| Achieve Novel Properties | Allows control over crystal orientation, defects, and grain size for tailored functionalities. |
| Lower Deposition Temperatures | Reduces energy use and enables coating of temperature-sensitive substrates like polymers. |
| Improve Safety and Sustainability | Uses less toxic, 'green' precursors to minimize hazards and environmental impact. |
| Enhance Process Control | Ensures reproducibility with stable vapor pressure and compatibility with modern furnace systems. |
Ready to elevate your thin-film research with cutting-edge CVD solutions? At KINTEK, we leverage exceptional R&D and in-house manufacturing to provide advanced high-temperature furnace systems, including Tube Furnaces and CVD/PECVD Systems, tailored to your unique needs. Our strong deep customization capability ensures precise alignment with your experimental goals—whether you're developing new precursors or scaling up processes. Contact us today to discuss how our expertise can drive your innovations forward!
Visual Guide
Related Products
- Custom Made Versatile CVD Tube Furnace Chemical Vapor Deposition CVD Equipment Machine
- Multi Heating Zones CVD Tube Furnace Machine for Chemical Vapor Deposition Equipment
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
People Also Ask
- Why is the tube design important in CVD furnaces? Ensure Uniform Deposition for High-Quality Films
- Why are advanced materials and composites important? Unlock Next-Gen Performance in Aerospace, Auto, and More
- What temperature ranges can a CVD Tube Furnace achieve with different tube materials? Unlock High-Temp Precision for Your Lab
- What types of atmosphere control does a CVD Tube Furnace support? Master Vacuum and Gas Control for Precision
- What makes a CVD Tube Furnace essential for material science and nanotechnology? Unlock Precision in Material Synthesis



















