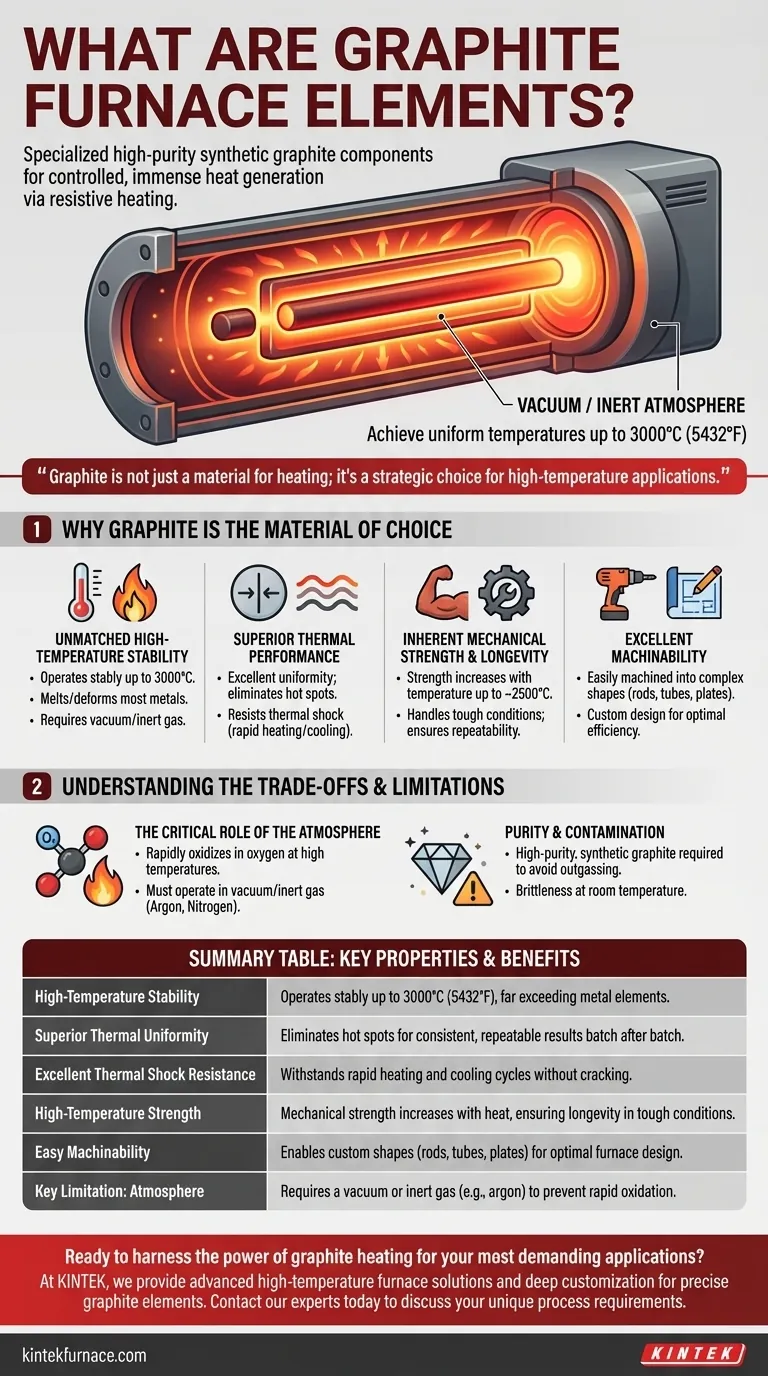
At their core, graphite furnace elements are specialized components made from high-purity synthetic graphite designed to generate immense heat within controlled environments. They function through resistive heating, where an electric current passes through the graphite, causing it to heat up and radiate energy. This allows industrial furnaces to achieve extremely high and uniform temperatures that are impossible with conventional metal elements.
Graphite is not just a material for heating; it's a strategic choice for high-temperature applications. Its unique combination of extreme temperature resistance, thermal stability, and machinability makes it the definitive material for creating uniform and repeatable heating conditions in demanding industrial furnaces.

Why Graphite is the Material of Choice
The selection of graphite is a deliberate engineering decision rooted in a unique set of physical properties that make it ideal for extreme thermal processing.
Unmatched High-Temperature Stability
Graphite elements can operate stably at temperatures up to 3000°C (5432°F). This capability far exceeds that of most metals, which would melt or deform under such conditions.
This performance, however, is only possible in a vacuum or an inert gas atmosphere (like argon or nitrogen).
Superior Thermal Performance
Graphite provides excellent temperature uniformity. It heats evenly and radiates energy consistently across its surface, eliminating hot spots that can ruin sensitive processes.
It also has a strong resistance to thermal shock. This means it can withstand rapid heating and cooling cycles without cracking or degrading, which is critical for industrial productivity.
Inherent Mechanical Strength and Longevity
Unlike metals that soften when heated, graphite's mechanical strength actually increases with temperature up to around 2500°C.
This robustness allows graphite elements to handle tough industrial conditions consistently over a long service life, ensuring process repeatability from one batch to the next.
Excellent Machinability
Despite its strength, graphite is relatively easy to machine. This allows for the creation of complex and precise element shapes, such as rods, tubes, or intricate plates.
This design flexibility is crucial for engineering a furnace's heating zone for optimal efficiency and thermal uniformity tailored to a specific application.
Understanding the Trade-offs and Limitations
While graphite is a superior material, its application requires acknowledging key operational constraints. Ignoring these trade-offs is the most common source of element failure.
The Critical Role of the Atmosphere
The single most important limitation of graphite is its reaction with oxygen. At high temperatures, graphite will rapidly oxidize (burn away) in the presence of air.
Therefore, graphite elements must be operated in a vacuum or be constantly bathed in an inert gas. A leak in the furnace system can lead to the rapid destruction of the elements.
Purity and Contamination
The performance described relies on the use of high-purity, synthetic graphite. Lower-grade materials or impurities can lead to outgassing, which contaminates the furnace atmosphere and the product being treated.
This high purity requirement can also influence the overall cost of the furnace's hot zone.
Brittleness at Room Temperature
While strong when hot, graphite can be brittle and fragile at room temperature. Care must be taken during furnace assembly, maintenance, and loading to avoid chipping or cracking the elements.
Applying This to Your High-Temperature Process
Your choice to use or specify a furnace with graphite elements should be guided by your primary process goal.
- If your primary focus is maximum temperature and uniformity: Graphite elements are the industry standard for processes exceeding 1500°C, providing unparalleled thermal stability.
- If you are designing or specifying a furnace: The ease of machining graphite allows for custom element designs tailored to your specific heating chamber for optimal efficiency.
- If your concern is operational reliability: You must invest in robust vacuum or inert gas systems, as protecting graphite elements from oxygen is the single most important factor for ensuring their longevity.
Understanding these principles empowers you to not only operate your equipment effectively but to specify and maintain it for maximum performance and lifespan.
Summary Table:
| Key Property | Benefit for Your Process |
|---|---|
| High-Temperature Stability | Operates stably up to 3000°C (5432°F), far exceeding metal elements. |
| Superior Thermal Uniformity | Eliminates hot spots for consistent, repeatable results batch after batch. |
| Excellent Thermal Shock Resistance | Withstands rapid heating and cooling cycles without cracking. |
| High-Temperature Strength | Mechanical strength increases with heat, ensuring longevity in tough conditions. |
| Easy Machinability | Enables custom shapes (rods, tubes, plates) for optimal furnace design. |
| Key Limitation: Atmosphere | Requires a vacuum or inert gas (e.g., argon) to prevent rapid oxidation. |
Ready to harness the power of graphite heating for your most demanding applications?
At KINTEK, we leverage our exceptional R&D and in-house manufacturing to provide advanced high-temperature furnace solutions. Our product line, including Tube Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems, is complemented by our strong deep customization capability. We can design and machine precise graphite elements to create the uniform, high-temperature environment your unique process requires.
Contact our experts today to discuss how a custom KINTEK furnace with graphite elements can achieve superior thermal performance and reliability for your lab.
Visual Guide
Related Products
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- Laboratory Muffle Oven Furnace with Bottom Lifting
- Split Multi Heating Zone Rotary Tube Furnace Rotating Tube Furnace
People Also Ask
- What is the primary function of a vacuum graphite furnace? Achieve Extreme-Temperature Material Purity
- What is the mechanism and effect of post-annealing NiTi thin films in a vacuum furnace? Unlock Superelasticity
- What is the significance of vacuum in relation to graphite components in furnaces? Prevent Oxidation for Extreme Temperatures
- How does vacuum heat treatment reduce workpiece deformation? Achieve Superior Dimensional Stability
- What is the primary application of vacuum heat treating furnaces in aerospace? Enhance Component Performance with Precision



















