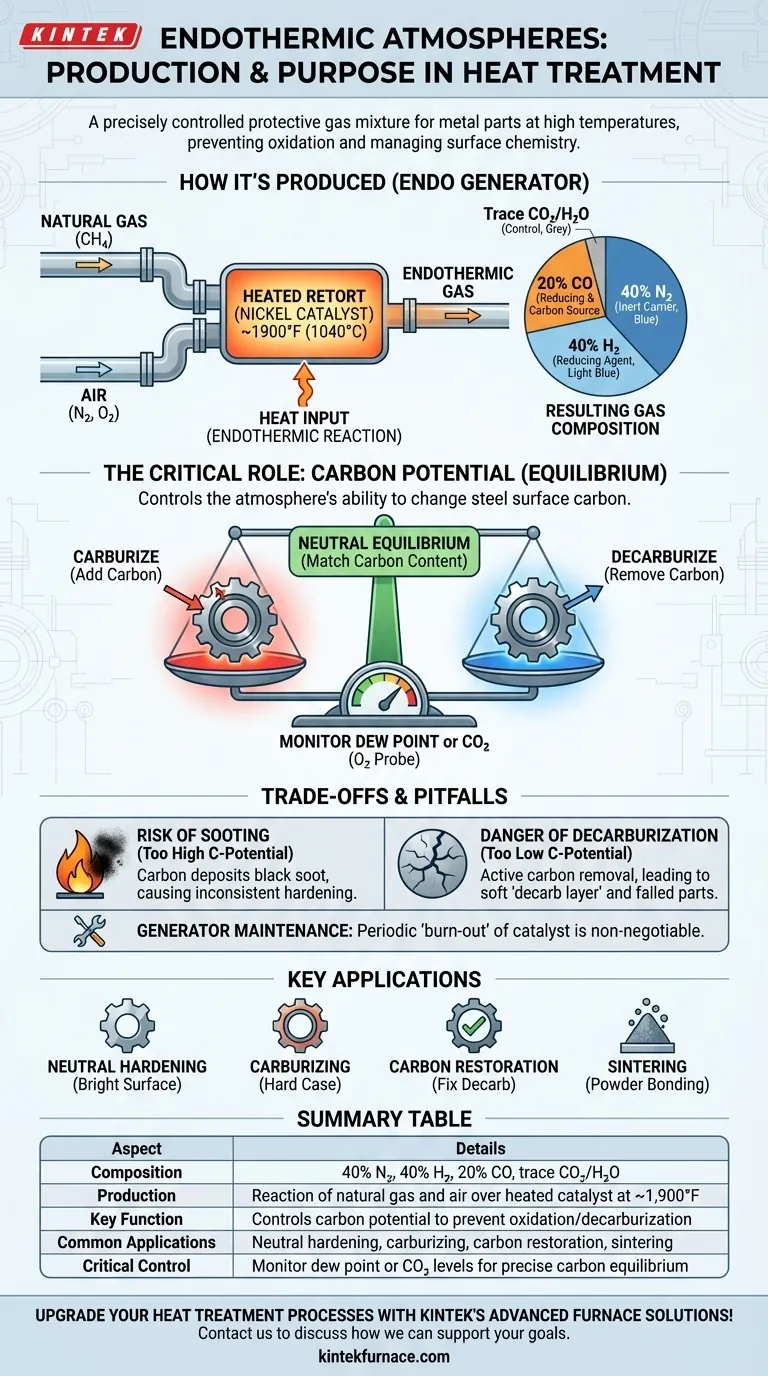
In the world of industrial heat treatment, an endothermic atmosphere is a precisely controlled gas mixture used to protect metal parts from oxidation and to manage their surface chemistry at high temperatures. It is produced inside an "endo generator" by reacting natural gas and air over a heated catalyst, creating a gas composed primarily of nitrogen, hydrogen, and carbon monoxide.
An endothermic atmosphere is not just a protective blanket for steel; it's an active chemical tool. By precisely controlling its composition, you can prevent oxidation and decarburization, ensuring the final material meets exact hardness and surface integrity specifications.

How an Endothermic Generator Works
The production of this atmosphere is a continuous chemical reaction that requires a dedicated piece of equipment.
The Core Reaction
An endothermic generator mixes a specific ratio of hydrocarbon gas (typically natural gas, which is mostly methane) and air. This mixture is then passed through a heated chamber, or retort, filled with a nickel-bearing catalyst at temperatures around 1,900°F (1,040°C).
The "Endothermic" Principle
The term endothermic means the reaction requires a constant input of heat to continue. The generator must continuously heat the retort to sustain the chemical cracking of the gas molecules. This is the opposite of an exothermic reaction, which releases heat.
The Resulting Gas Composition
This process "cracks" the hydrocarbon and air into a new mixture. A typical endothermic atmosphere consists of approximately:
- 40% Nitrogen (N₂): An inert carrier gas from the air.
- 40% Hydrogen (H₂): A strong reducing agent that actively removes oxygen.
- 20% Carbon Monoxide (CO): A reducing agent that also provides carbon for the atmosphere.
- Trace amounts of Carbon Dioxide (CO₂) and Water Vapor (H₂O): These are critical for process control.
The Critical Role of Carbon Potential
The true function of an endothermic atmosphere goes far beyond simple protection. Its primary purpose is to control the carbon equilibrium between the furnace atmosphere and the steel's surface.
Defining Carbon Potential
Carbon potential is the ability of the atmosphere to change the carbon concentration on the surface of the steel. The atmosphere can either add carbon (carburize), remove it (decarburize), or remain perfectly neutral.
Achieving Equilibrium
The goal of most "neutral hardening" processes is to heat and cool the part without changing its surface chemistry. To do this, the carbon potential of the gas must be matched exactly to the carbon content of the alloy being treated.
How It's Controlled
The carbon potential is determined by the ratios of gases in the atmosphere, specifically CO/CO₂ and H₂/H₂O. By monitoring and controlling the dew point (a measure of water vapor) or the CO₂ content with an oxygen probe, an operator can precisely "dial in" the atmosphere for a specific steel.
Understanding the Trade-offs and Pitfalls
Improperly generated or controlled endothermic gas is a primary cause of heat-treating defects.
The Risk of Sooting
If the carbon potential is set too high for the temperature, the carbon monoxide will break down and deposit black carbon soot on the parts, furnace fixtures, and insulation. This creates a mess and can lead to inconsistent hardening.
The Danger of Decarburization
If the atmosphere's carbon potential is too low (often due to air leaks or an exhausted generator catalyst), it will actively pull carbon out of the steel's surface. This soft "decarb layer" will not harden properly during quenching, resulting in a failed part.
Generator Maintenance is Non-Negotiable
The catalyst inside the generator retort has a finite life. It becomes coated with soot over time and must be periodically "burned out" with air to clean and reactivate it. Failure to perform this maintenance leads to poor quality gas and unpredictable results.
Key Applications in Heat Treatment
Endothermic gas is the workhorse atmosphere for carbon-based steels and is used in several key processes.
Neutral Hardening (Bright Hardening)
The most common application. The goal is to heat a steel part for hardening without scaling (oxidizing) or changing the surface carbon content. The finished part retains a clean, bright surface.
Carburizing
Here, the atmosphere's carbon potential is intentionally set much higher than the steel's base carbon. This forces carbon to diffuse into the surface of a low-carbon steel part, creating a hard, wear-resistant "case" around a softer, tougher core.
Carbon Restoration
This process is used to fix parts that were accidentally decarburized during prior manufacturing steps like forging. A correctly controlled endothermic atmosphere restores the lost carbon to the surface before the final hardening.
Sintering
In powder metallurgy, endothermic gas provides a protective environment to heat compacted metal powders, allowing the particles to bond together into a solid object without oxidizing.
Making the Right Choice for Your Process
After ensuring your generator is healthy, you must match the atmosphere to your metallurgical goal.
- If your primary focus is neutral hardening: Your goal is precise equilibrium; monitor dew point or CO₂ levels constantly to match the atmosphere's carbon potential to your steel.
- If your primary focus is case carburizing: You will operate with a higher carbon potential, but must be vigilant about preventing excessive sooting in the furnace and on the parts.
- If you are experiencing inconsistent results: Immediately audit your endo-generator's health, checking for a declining catalyst, improper gas/air ratios, or air leaks in the furnace.
Mastering your endothermic atmosphere is fundamental to achieving consistent and reliable heat treatment outcomes.
Summary Table:
| Aspect | Details |
|---|---|
| Composition | 40% N₂, 40% H₂, 20% CO, trace CO₂/H₂O |
| Production | Reaction of natural gas and air over heated catalyst at ~1,900°F |
| Key Function | Controls carbon potential to prevent oxidation/decarburization |
| Common Applications | Neutral hardening, carburizing, carbon restoration, sintering |
| Critical Control | Monitor dew point or CO₂ levels for precise carbon equilibrium |
Upgrade your heat treatment processes with KINTEK's advanced furnace solutions! Leveraging exceptional R&D and in-house manufacturing, we provide diverse laboratories with high-temperature furnaces like Muffle, Tube, Rotary, Vacuum & Atmosphere, and CVD/PECVD Systems. Our deep customization capabilities ensure precise alignment with your unique experimental needs, enhancing efficiency and reliability. Contact us today to discuss how we can support your goals in metal heat treatment and beyond!
Visual Guide
Related Products
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
People Also Ask
- What is the main purpose of heat treatment? Transform Metal Properties for Superior Performance
- What is nitrogen used for in a furnace? Prevent Oxidation and Control Heat Treatment Quality
- How does a chemically inert atmosphere function in a furnace? Prevent Oxidation and Ensure Material Purity
- What is the use of nitrogen in furnace? Prevent Oxidation for Superior Heat Treatment
- How does an inert atmosphere prevent oxidation? Shield Materials from Oxygen Damage



















