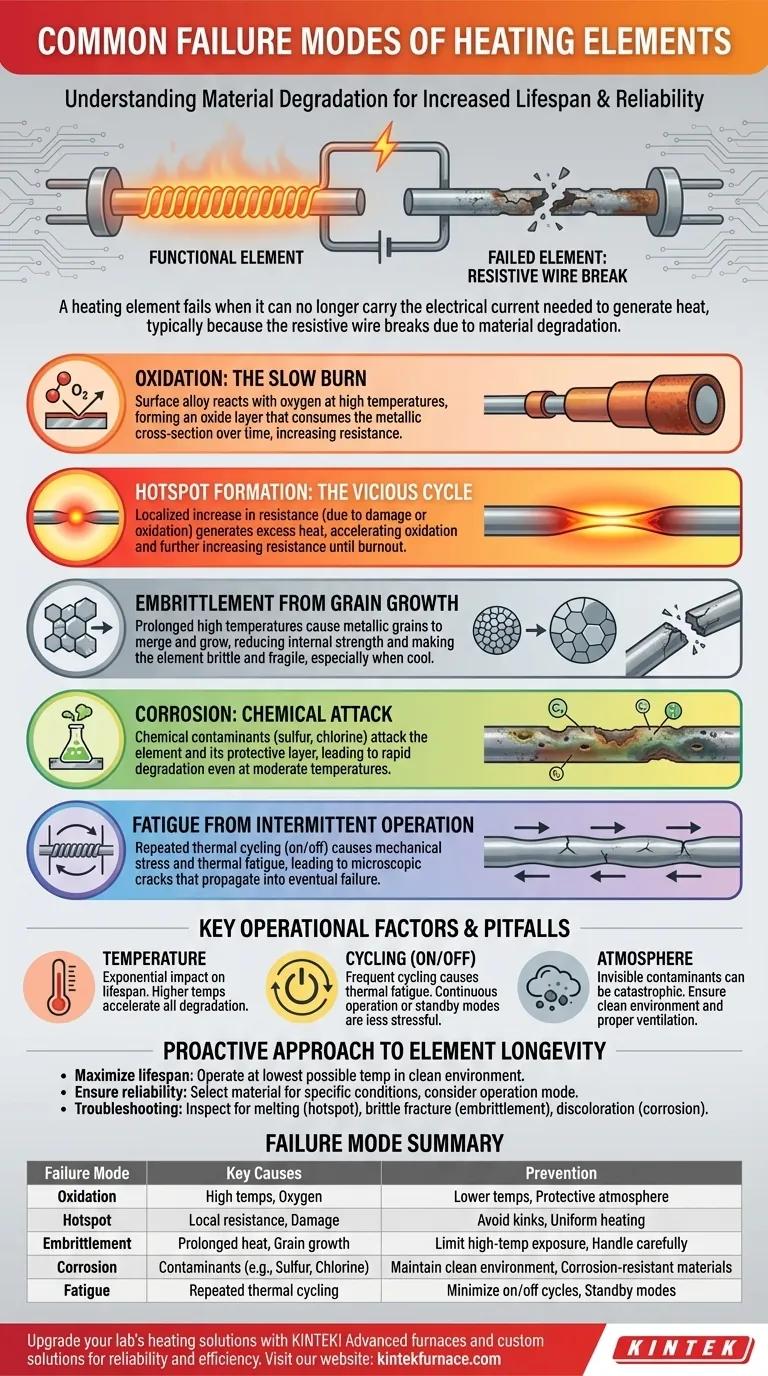
At its core, a heating element fails when it can no longer carry the electrical current needed to generate heat, typically because the resistive wire breaks. The most common causes of this break are a combination of material degradation processes, including oxidation, the formation of localized hotspots, embrittlement from grain growth, fatigue from repeated cycling, and chemical corrosion.
Understanding heating element failure is not about predicting a random event. It is about understanding a predictable process of material degradation, where operating temperature, atmospheric conditions, and operational cycles are the primary drivers of an element's lifespan.

The Science of Material Degradation
A heating element's failure is almost always the final step in a longer process of material breakdown. By understanding these mechanisms, you can diagnose failures and take steps to prevent them.
Oxidation: The Slow Burn
At high temperatures, the surface of the element alloy reacts with oxygen in the air. This process, known as oxidation, forms a thin, protective oxide layer.
For quality elements like those made from Nichrome (nickel-chromium), this layer is stable and self-healing, protecting the metal underneath. However, over time and at extreme temperatures, this process consumes the metallic cross-section of the wire, increasing its electrical resistance.
Hotspot Formation: The Vicious Cycle
A hotspot is a localized area on the element that runs significantly hotter than its surroundings. This is one of the most common and destructive failure modes.
Hotspots are caused by a local increase in resistance. This can happen if the element is damaged, kinked, or if oxidation has thinned a small section of the wire.
This creates a feedback loop: higher local resistance generates more heat, which accelerates oxidation in that spot, which further thins the wire and increases resistance. This cycle continues rapidly until the wire melts or burns through at the hotspot.
Embrittlement from Grain Growth
Heating elements are made of crystalline metal alloys. When held at high temperatures for extended periods, the individual metallic grains within the alloy can merge and grow larger.
This grain growth reduces the material's internal strength and ductility. The element becomes brittle, especially after it cools down. A brittle element is extremely fragile and can easily fracture from minor vibrations, mechanical shock, or the stress of its own expansion and contraction.
Corrosion: Chemical Attack
While oxidation is a reaction with oxygen, corrosion is a chemical attack from other contaminants in the atmosphere.
Substances like sulfur, chlorine, and various metallic vapors can aggressively attack the element and its protective oxide layer, even at moderate temperatures. This leads to rapid degradation and premature failure, often far below the element's rated temperature limits.
Fatigue from Intermittent Operation
Every time an element is turned on and off, it undergoes thermal expansion and contraction. This repeated cycling puts mechanical stress on the wire.
This stress can cause microscopic cracks to form and grow, particularly in the protective oxide layer. This is known as thermal fatigue. Over thousands of cycles, these cracks can propagate through the element itself, leading to an eventual break.
Key Operational Factors and Pitfalls
The way you operate a heating element has a more significant impact on its lifespan than almost any other factor.
The Double-Edged Sword of Temperature
The relationship between operating temperature and element life is exponential. A seemingly small increase in temperature can cut the element's lifespan in half or more.
Running an element hotter provides faster heat-up times, but it dramatically accelerates oxidation, grain growth, and the risk of hotspots.
The Impact of Cycling (On/Off)
For many materials, continuous operation at a stable temperature is less stressful than frequent on/off cycles.
If your process allows, holding a system at a lower standby temperature can be better for element longevity than cycling it completely off and on. This minimizes the expansion-contraction stress that causes thermal fatigue.
The Critical Role of the Atmosphere
Never underestimate the impact of the operating environment. Contaminants that are invisible to the eye can be catastrophic for a heating element.
Ensure the area is free from cutting fluids, cleaning agents, or process byproducts that could introduce corrosive agents into the atmosphere. Proper ventilation is critical in environments where contamination is unavoidable.
A Proactive Approach to Element Longevity
To extend the life of your heating elements, shift your focus from reacting to failures to proactively controlling the conditions that cause them.
- If your primary focus is maximizing lifespan: Operate the element at the lowest possible temperature that still achieves your goal and ensure the operating environment is clean and free of chemical contaminants.
- If your primary focus is ensuring reliability: Select an element material specifically designed for your temperature range and atmosphere, and carefully consider whether continuous or intermittent operation is more suitable for your process.
- If your primary focus is troubleshooting a failure: Carefully inspect the broken element for clues. A melted, necked-down break suggests a hotspot, while a clean, brittle fracture points to embrittlement, and discoloration or pitting may indicate corrosion.
Understanding why an element fails is the first and most critical step toward preventing that failure in the future.
Summary Table:
| Failure Mode | Key Causes | Prevention Tips |
|---|---|---|
| Oxidation | High temperatures, oxygen exposure | Operate at lower temps, use protective atmospheres |
| Hotspot Formation | Local resistance increases, damage | Avoid kinks, ensure uniform heating |
| Embrittlement | Grain growth from prolonged heat | Limit high-temp exposure, handle carefully when cool |
| Corrosion | Chemical contaminants (e.g., sulfur, chlorine) | Maintain clean environment, use corrosion-resistant materials |
| Fatigue | Repeated thermal cycling | Minimize on/off cycles, consider standby modes |
Upgrade your lab's heating solutions with KINTEK! Leveraging exceptional R&D and in-house manufacturing, we provide advanced high-temperature furnaces like Muffle, Tube, Rotary, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems. Our deep customization capabilities ensure precise fits for your unique experimental needs, enhancing reliability and efficiency. Contact us today to discuss how we can help prevent heating element failures and optimize your processes!
Visual Guide
Related Products
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- Multi Zone Laboratory Quartz Tube Furnace Tubular Furnace
People Also Ask
- What role does a muffle furnace play in the preparation of MgO support materials? Master Catalyst Activation
- What substances are prohibited from being introduced into the furnace chamber? Prevent Catastrophic Failure
- What is the role of a muffle furnace in the study of biochar regeneration and reuse? Unlock Sustainable Water Treatment
- How does a laboratory muffle furnace facilitate the biomass carbonization process? Achieve Precise Biochar Production
- What is the role of a muffle furnace in the synthesis of water-soluble Sr3Al2O6? Precision in SAO Production



















