At its core, the environment for Chemical Vapor Deposition (CVD) is created by introducing specific reactive gases, known as precursors, into a sealed reaction chamber containing the object to be coated. This chamber's internal conditions—primarily temperature and pressure—are precisely controlled to trigger a chemical reaction, causing a solid material to form and deposit as a thin film onto the object's surface.
The creation of a CVD environment is not a single action but a carefully orchestrated sequence. It involves isolating a substrate in a controlled chamber, introducing precise amounts of volatile chemical precursors, and applying a specific form of energy (typically heat) to drive a surface reaction that builds the desired film.

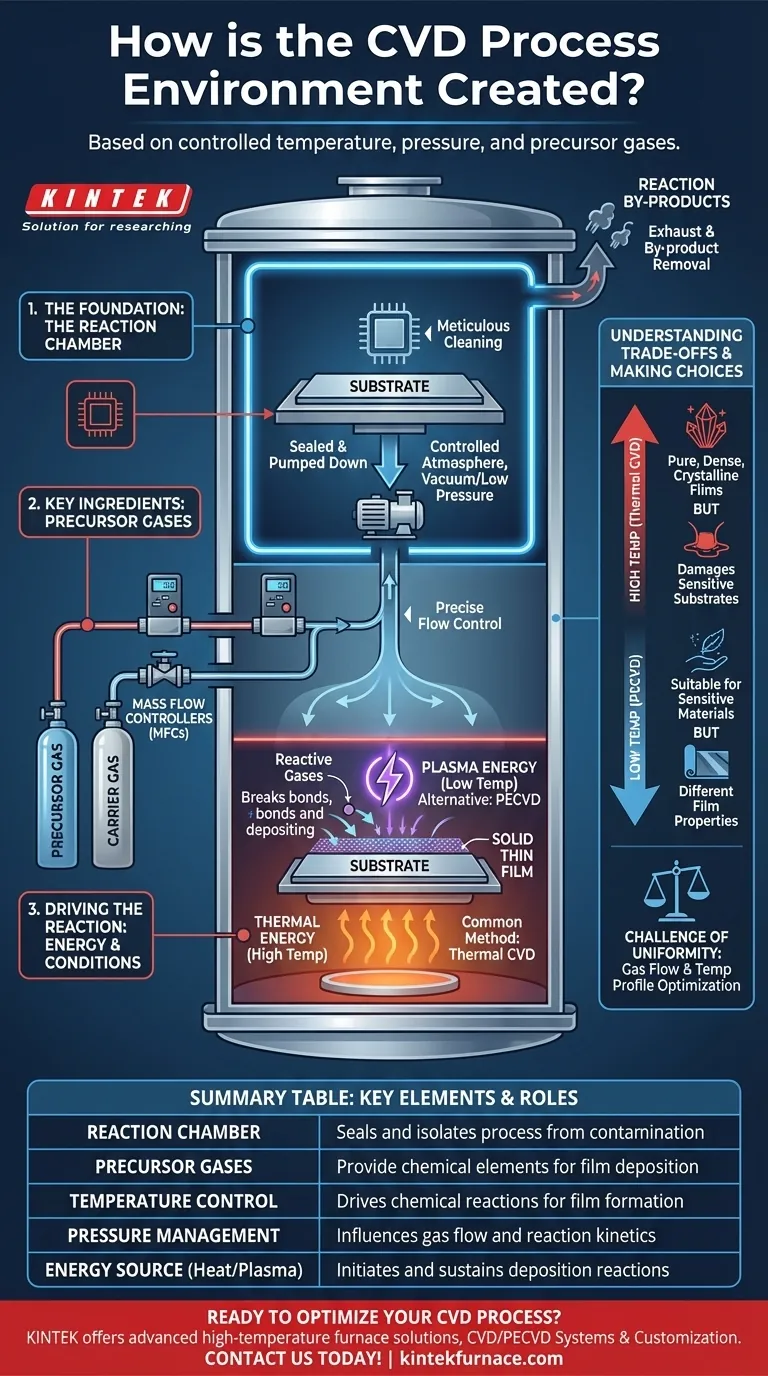
The Foundation: The Reaction Chamber
The entire CVD process occurs within a highly controlled physical enclosure. The quality and control of this chamber environment are the first and most critical factors for success.
Sealing the Enclosure
The process begins with placing the parts or materials to be coated, known as substrates, inside a sealed reaction chamber. This enclosure isolates the process from the outside atmosphere, preventing contamination from oxygen, water vapor, and other particles.
Substrate Preparation
Before being sealed in the chamber, the substrate must be meticulously cleaned. Any surface contaminants can inhibit the chemical reaction or become impurities in the final film, compromising its quality and performance.
Establishing a Controlled Atmosphere
Once sealed, the chamber is typically pumped down to a vacuum or a specific low pressure. This step serves two purposes: it removes any residual air and contaminants, and it creates a controlled baseline pressure before the reactive gases are introduced.
The Key Ingredients: Precursor Gases
With the chamber prepared, the specific chemical ingredients for the film are introduced. These are not added randomly but with extreme precision.
What Are Precursors?
Precursors are volatile chemical compounds, either gases or vaporized liquids, that contain the specific elements needed for the final film. For example, to create a silicon nitride film, precursors containing silicon (like silane) and nitrogen (like ammonia) would be used.
The Role of Carrier Gases
Often, the precursor gases are too concentrated to be used directly. They are mixed with an inert carrier gas, such as argon or nitrogen. This carrier gas helps transport the precursors into the chamber at a stable, controlled rate without participating in the chemical reaction itself.
Precise Flow Control
The exact amount of each gas entering the chamber is managed by devices called mass flow controllers (MFCs). This precise control over the gas mixture is essential for determining the final chemical composition and properties of the deposited film.
Driving the Reaction: Energy and Conditions
Simply mixing gases in a chamber isn't enough. Energy must be supplied to break the chemical bonds in the precursor molecules and initiate the deposition reaction.
The Critical Role of Temperature
In the most common method, Thermal CVD, the substrate is heated to a specific, often very high, temperature. This thermal energy energizes the precursor molecules when they arrive at the hot surface, causing them to react and deposit the solid film. The temperature is one of the most critical variables affecting the film's structure and growth rate.
Managing Pressure
The pressure inside the chamber is carefully maintained throughout the process. Pressure influences how gases flow, the concentration of reactants at the substrate surface, and whether reactions occur primarily on the surface or in the gas phase above it.
Removing Reaction By-products
The chemical reaction that forms the solid film also creates unwanted gaseous by-products. A continuous, gentle gas flow, managed by the vacuum and exhaust system, removes these by-products from the chamber. If not removed, they could contaminate the film or slow down the deposition process.
Understanding the Trade-offs
Creating the ideal CVD environment is a balancing act. The choices made directly impact the outcome and are dictated by the material being deposited and the substrate it's being coated on.
High vs. Low Temperature
High temperatures, used in thermal CVD, typically produce very pure, dense, and crystalline films. However, they cannot be used on substrates that would melt or be damaged by the heat, such as plastics or certain electronic components.
The Need for Energy Alternatives
For temperature-sensitive substrates, alternative methods like Plasma-Enhanced CVD (PECVD) are used. In PECVD, an electric field creates a plasma in the chamber. This plasma provides the energy to drive the reaction, allowing deposition to occur at much lower temperatures. The trade-off is that these films may have different properties than their high-temperature counterparts.
The Challenge of Uniformity
Ensuring the film deposits at the same thickness across the entire substrate is a major engineering challenge. It requires optimizing gas flow dynamics, maintaining a uniform temperature profile, and preventing the precursor gases from being used up before they reach the far side of the substrate.
Making the Right Choice for Your Goal
The specific environmental setup is always tailored to the desired outcome. Understanding the levers you can pull is key to achieving the right film properties.
- If your primary focus is creating a high-purity, crystalline film: You must prioritize a high-vacuum chamber, high-purity precursors, and the high temperatures associated with thermal CVD.
- If your primary focus is coating a temperature-sensitive material: Your environment must be based on a low-temperature process like PECVD, where plasma provides the reaction energy instead of heat.
- If your primary focus is precise control over film composition: You must invest in highly accurate mass flow controllers and ensure stable, repeatable control over both pressure and temperature throughout the deposition.
Ultimately, mastering the CVD process is synonymous with mastering the control of its environment.
Summary Table:
| Key Element | Role in CVD Environment |
|---|---|
| Reaction Chamber | Seals and isolates the process to prevent contamination |
| Precursor Gases | Provide chemical elements for film deposition |
| Temperature Control | Drives chemical reactions for film formation |
| Pressure Management | Influences gas flow and reaction kinetics |
| Energy Source (e.g., Heat or Plasma) | Initiates and sustains deposition reactions |
Ready to optimize your CVD process with tailored solutions? KINTEK leverages exceptional R&D and in-house manufacturing to provide advanced high-temperature furnace solutions, including CVD/PECVD Systems, Muffle, Tube, Rotary, Vacuum & Atmosphere Furnaces. Our strong deep customization capability ensures we meet your unique experimental needs—contact us today to enhance your lab's performance!
Visual Guide
Related Products
- Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
- Inclined Rotary Plasma Enhanced Chemical Deposition PECVD Tube Furnace Machine
- Custom Made Versatile CVD Tube Furnace Chemical Vapor Deposition CVD Equipment Machine
- Multi Heating Zones CVD Tube Furnace Machine for Chemical Vapor Deposition Equipment
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
People Also Ask
- What are the advantages of using CVD? Achieve High-Purity, Conformal Thin Films for Your Applications
- What is PECVD specification? A Guide to Choosing the Right System for Your Lab
- How is silicon dioxide deposited from tetraethylorthosilicate (TEOS) in PECVD? Achieve Low-Temperature, High-Quality SiO2 Films
- What are the classifications of CVD based on vapor characteristics? Optimize Your Thin Film Deposition Process
- What is plasma-deposited silicon nitride, and what are its properties? Discover Its Role in Solar Cell Efficiency



















