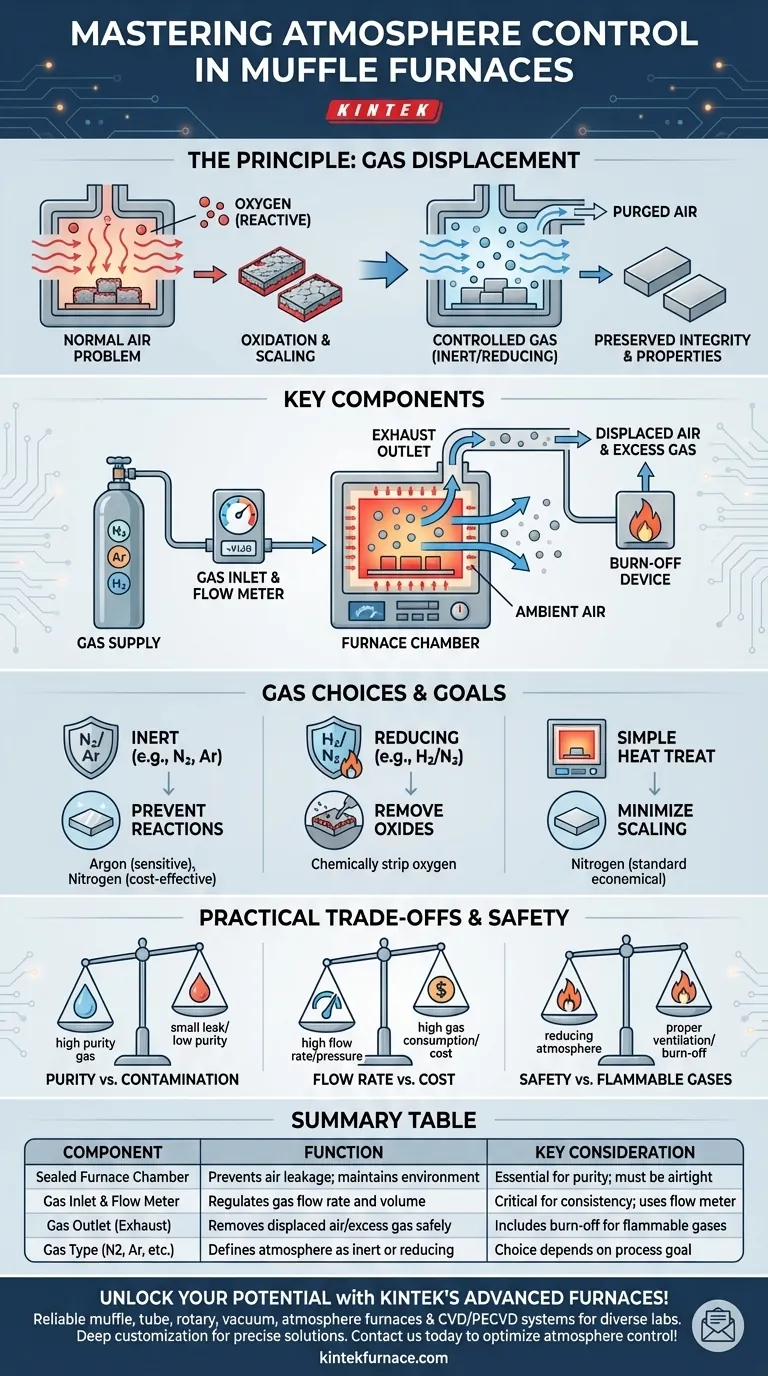
In an atmosphere protection muffle furnace, the atmosphere is controlled by continuously feeding a specific gas into the sealed furnace chamber during operation. This introduced gas displaces the ambient air, particularly oxygen, creating a precisely managed environment. A gas flow meter is the primary tool used to regulate the volume and rate of this gas, ensuring a stable and consistent atmosphere throughout the heat treatment process.
The core purpose of atmosphere control is not just to add a gas, but to actively displace the reactive oxygen in the air. This prevents unwanted chemical changes like oxidation, allowing materials to be heated to very high temperatures while preserving their integrity and desired properties.

The Principle: Preventing Unwanted Reactions
At the high temperatures achieved in a muffle furnace, most materials become highly reactive with the oxygen present in normal air. This fundamental problem is what atmosphere control is designed to solve.
Why Normal Air is a Problem
When heated in air, many metals will rapidly oxidize, forming a layer of scale on the surface. This can alter the material's dimensions, compromise its structural integrity, and ruin its surface finish.
For other sensitive processes like sintering, the presence of oxygen can interfere with the chemical bonding between particles, leading to failed or subpar results.
The Solution: Gas Displacement
Atmosphere control works on the principle of displacement. By flooding the sealed furnace chamber with a high-purity gas, you physically push out the ambient air.
This purge creates an environment that is either chemically non-reactive (inert) or actively beneficial to the process (reducing).
Key Components for Atmosphere Control
Achieving a stable, controlled atmosphere relies on a simple but critical system of components working together.
The Sealed Furnace Chamber
The entire process begins with an airtight or well-sealed furnace chamber. A proper seal is essential to prevent the controlled atmosphere from escaping and, more importantly, to stop outside air from leaking in and contaminating the process.
The Gas Inlet and Flow Meter
A specific gas (e.g., Nitrogen, Argon, Hydrogen) is supplied from a cylinder or generator and piped to an inlet on the furnace.
A gas flow meter is installed in this line. This device is the command center for atmosphere control, allowing the operator to set and maintain a precise, constant flow rate. This stability is crucial for ensuring the atmosphere remains consistent for the duration of the cycle.
The Gas Outlet (Exhaust)
As the controlled gas is fed into the chamber, the displaced air and any excess gas must have a way to exit. This is typically managed through a simple exhaust port or a pressure-relief valve. For flammable gases like hydrogen, this outlet often leads to a burn-off device to safely ignite the exiting gas.
Understanding the Practical Trade-offs
While the concept is straightforward, effective atmosphere control involves balancing several practical factors.
Purity vs. Contamination
The effectiveness of the process is directly tied to the purity of the gas and the integrity of the furnace seal. Even a small leak or the use of a low-purity gas can introduce enough oxygen to cause unwanted oxidation.
Flow Rate vs. Cost
A higher gas flow rate ensures a more thorough purge of the chamber and provides a positive pressure that helps prevent air from entering. However, this also increases the consumption of gas, leading to higher operational costs. The goal is to find the minimum flow rate that reliably protects the material.
Safety with Flammable Gases
Using a reducing atmosphere, such as a nitrogen/hydrogen mix, introduces a significant safety consideration. These systems require proper ventilation and a reliable ignition source at the exhaust to safely burn off the flammable hydrogen gas, preventing it from accumulating to explosive levels.
Making the Right Choice for Your Goal
The choice of gas is dictated entirely by the desired outcome of your heat treatment process.
- If your primary focus is preventing all reactions (inertness): Use an inert gas like high-purity Argon (for highly sensitive materials) or Nitrogen (a cost-effective choice for most general-purpose applications).
- If your primary focus is actively removing surface oxides (reducing): Use a reducing atmosphere, such as a blend of Hydrogen and Nitrogen, to chemically strip oxygen atoms from the material's surface.
- If your primary focus is simple heat treatment without heavy scaling: An inert atmosphere of Nitrogen is the standard and most economical choice for protecting the material.
Mastering atmosphere control gives you direct power over the final chemistry and properties of your materials.
Summary Table:
| Component | Function | Key Consideration |
|---|---|---|
| Sealed Furnace Chamber | Prevents air leakage and maintains controlled environment | Essential for purity; must be airtight |
| Gas Inlet and Flow Meter | Regulates gas flow rate and volume for stable atmosphere | Critical for consistency; uses flow meter |
| Gas Outlet (Exhaust) | Removes displaced air and excess gas safely | Includes burn-off for flammable gases |
| Gas Type (e.g., Nitrogen, Argon) | Defines atmosphere as inert or reducing | Choice depends on process goal (e.g., oxidation prevention) |
Unlock the full potential of your heat treatment processes with KINTEK's advanced high-temperature furnace solutions! Leveraging exceptional R&D and in-house manufacturing, we provide diverse laboratories with reliable muffle, tube, rotary, vacuum, atmosphere furnaces, and CVD/PECVD systems. Our strong deep customization capability ensures precise solutions for your unique experimental needs, from preventing oxidation to enhancing material properties. Contact us today to discuss how our expertise can optimize your atmosphere control and boost efficiency!
Visual Guide
Related Products
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- Laboratory Muffle Oven Furnace with Bottom Lifting
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
People Also Ask
- How does the inert atmosphere heat treating process work? Prevent Oxidation for Superior Material Quality
- What does inert mean in furnace atmospheres? Protect materials from oxidation with inert gases.
- What are the benefits of inert atmosphere heat treating? Prevent Oxidation and Preserve Material Integrity
- How does nitrogen atmosphere heat treatment improve surface strengthening? Enhance Durability and Performance
- What is nitrogen used for in a furnace? Prevent Oxidation and Control Heat Treatment Quality



















