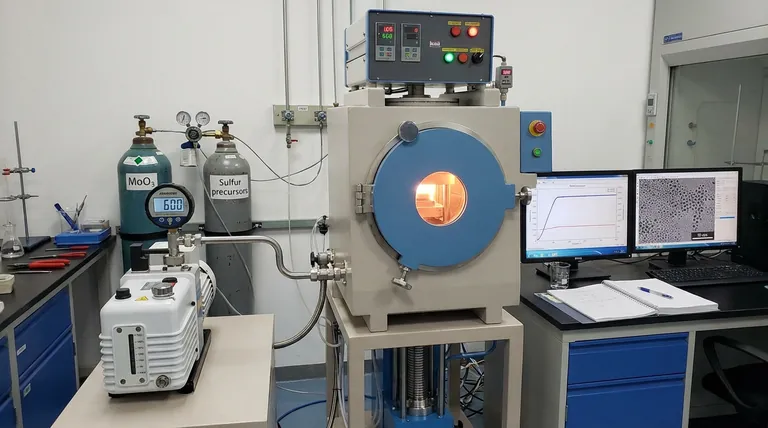
A vacuum pump is the critical control mechanism that enables the formation of Type III MoS2 dot structures by maintaining a specific low-pressure environment. By working in tandem with valves to stabilize the chamber at approximately 600 mTorr, the pump creates the thermodynamic conditions necessary for precursor vaporization and the physical retraction of material into distinct nanoparticles.
Core Takeaway The vacuum pump serves a dual purpose: it drives the full vaporization of MoO3 precursors and facilitates a "dewetting" phenomenon on the substrate. This specific pressure environment forces the growing material to shrink into discrete 20-30 nm droplets rather than spreading into a continuous film.
The Role of Pressure in Growth Dynamics
Controlling the Environment
The formation of Type III structures is not spontaneous; it requires a highly controlled atmosphere.
The vacuum pump, regulating the system via valves, holds the pressure at a precise 600 mTorr. This specific pressure point is the foundational requirement for the subsequent chemical and physical reactions.
Facilitating Precursor Vaporization
At standard atmospheric pressures, molybdenum oxide (MoO3) precursors may not behave as required for this specific growth mode.
The low-pressure environment generated by the pump promotes the full vaporization of these precursors. This ensures that the reactants are in the correct gas phase to deposit effectively onto the target surface.
Inducing Material Shrinkage
The defining characteristic of Type III structures is their "dot-like" morphology.
The 600 mTorr environment leverages the poor wettability of MoS2 on WS2 surfaces. Because the pressure conditions discourage the material from spreading flat, the MoS2 is physically forced to shrink and bead up.
The Sulfurization Result
This shrinkage occurs specifically during the sulfurization phase of the growth process.
As the material retracts due to the low-pressure and wettability dynamics, it forms 20-30 nm droplet-like particles. These discrete particles are what constitute the final Type III MoS2/WS2 heterostructure.
Understanding the Trade-offs
Precision is Non-Negotiable
While a vacuum pump is a standard tool, the requirement here is not simply "as low as possible."
The process relies on a precise 600 mTorr environment. Deviating significantly from this pressure could alter the vaporization rate of the MoO3 or change the surface tension dynamics, potentially failing to form the distinct dots.
Surface Interaction Dependencies
The pump enables the formation of dots, but it relies on the underlying material properties to work.
This method specifically exploits the interaction between MoS2 and WS2. The vacuum pump enhances the natural tendency of MoS2 to dewet from WS2; this technique may not be transferable to material pairings that have high wettability (where materials naturally stick and spread).
Making the Right Choice for Your Goal
Achieving Type III MoS2 dot structures requires strict adherence to pressure parameters. Use the following guide to align your process setup:
- If your primary focus is obtaining discrete Dot Structures (Type III): You must calibrate your vacuum pump and valves to lock pressure specifically at 600 mTorr to trigger the necessary material shrinkage.
- If your primary focus is Precursor Efficiency: Ensure your pump maintains sufficiently low pressure to achieve full vaporization of MoO3, preventing unreacted solids from contaminating the substrate.
Success in growing Type III structures relies on using vacuum pressure not just to clear the chamber, but to physically shape the material at the nanoscale.
Summary Table:
| Feature | Parameter | Impact on MoS2 Growth |
|---|---|---|
| Target Pressure | 600 mTorr | Creates thermodynamic environment for dot formation |
| Precursor State | MoO3 Vaporization | Ensures gas-phase reactants deposit effectively |
| Morphology | Dewetting/Shrinkage | Forces material into 20-30 nm discrete droplets |
| Surface Type | Low-Wettability (WS2) | Facilitates the "beading up" effect of MoS2 |
| Phase Focus | Sulfurization Phase | Critical stage where material retracts into dots |
Elevate Your Nanoscale Research with KINTEK Precision
Achieving perfect Type III MoS2 dot structures requires more than just a vacuum—it requires absolute pressure stability. Backed by expert R&D and manufacturing, KINTEK offers high-performance Vacuum, CVD, and High-Temperature Furnace systems designed to meet the rigorous demands of material science.
Whether you need customizable solutions for MoO3 vaporization or precise pressure control for complex heterostructures, KINTEK provides the reliability your lab deserves. Contact us today to discover how our advanced lab equipment can optimize your growth process and drive your next breakthrough.
References
- Jungtae Nam, Keun‐Soo Kim. Tailored Synthesis of Heterogenous 2D TMDs and Their Spectroscopic Characterization. DOI: 10.3390/nano14030248
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Hot Press Furnace Machine for Lamination and Heating
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- 304 316 Stainless Steel High Vacuum Ball Stop Valve for Vacuum Systems
- Vacuum Heat Treat Sintering and Brazing Furnace
People Also Ask
- Why is a shell roasting furnace used prior to the pouring of C1023 superalloys? Expert Guide to Casting Integrity
- What level of temperature uniformity can be achieved in vacuum furnaces? Achieve ±2°C Precision for Critical Applications
- How is furnace brazing applied in research and development? A Precision Tool for Material Science & Prototyping
- Why is an industrial high vacuum sintering furnace required for high-porosity Hastelloy-X? Ensure Alloy Integrity
- How do vacuum furnace systems facilitate accelerated aging tests for SDSS2507? Ensure Precision in Neutron Scattering
- What role does carbonization in a high-temperature furnace play for 2D COF membranes? Enhance Stability & Conductivity
- What are the primary inert gases used in vacuum furnaces? Optimize Your Heat Treatment Process
- What are the main benefits of using a vacuum furnace? Achieve Purity and Precision in Heat Treatment



















