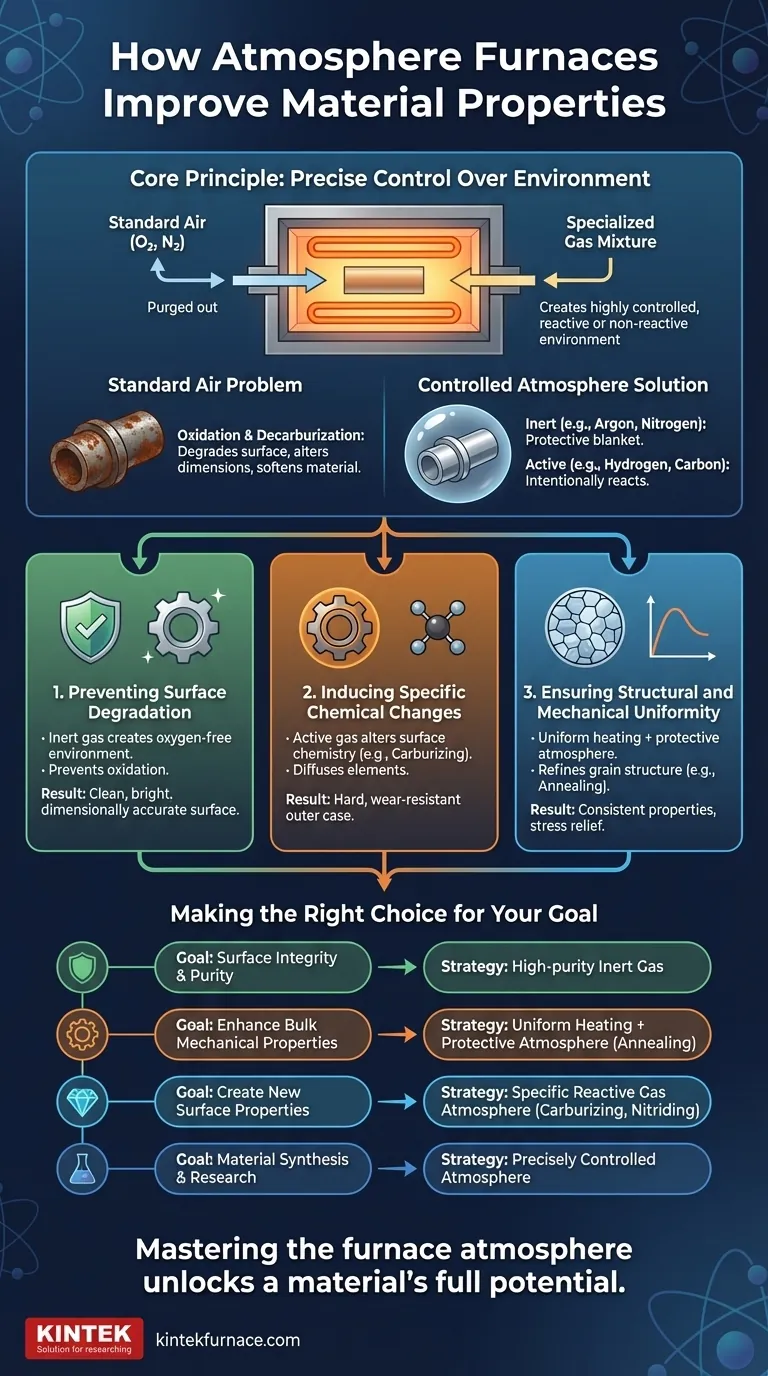
At their core, atmosphere furnaces improve materials by giving you precise control over chemistry and physics during heat treatment. Instead of simply heating a material in open air, these furnaces create a specialized gas environment. This control allows you to prevent destructive reactions like oxidation, intentionally introduce new elements into the material's surface, and ensure a uniform internal structure, resulting in superior strength, finish, and performance.
The primary value of an atmosphere furnace isn't just heating; it's creating a highly controlled, reactive or non-reactive environment. This allows you to dictate the final chemical and physical properties of a material, moving beyond simple hardening to true material engineering.

The Principle of Atmospheric Control
An atmosphere furnace replaces the ambient air (roughly 78% nitrogen, 21% oxygen) with a specific, highly-regulated gas mixture. This fundamental change is what unlocks advanced material properties.
Why Standard Air Is a Problem
Heating materials in open air is often detrimental. The oxygen present will readily react with a hot metal surface, causing oxidation (scaling or rust) which degrades the surface finish and can alter component dimensions. For certain steels, the oxygen can also react with carbon near the surface, a process called decarburization, which softens the material and reduces its fatigue life.
The Role of Controlled Atmospheres
By purging the furnace chamber of air and filling it with a specific gas, you take control of the chemistry. These atmospheres generally fall into two categories:
- Inert Atmospheres: Gases like argon or nitrogen are used. They are non-reactive and serve primarily to displace oxygen, creating a protective blanket around the part.
- Active Atmospheres: These are gas mixtures designed to intentionally react with the material. Examples include hydrogen-rich atmospheres (reducing), carbon-rich atmospheres (carburizing), or precisely controlled oxygen levels for specific synthesis processes.
Key Mechanisms for Material Improvement
Controlling the atmosphere enables three distinct improvements: protecting the material, changing the material, and perfecting the material's internal structure.
Preventing Surface Degradation
The most common use of a controlled atmosphere is protection.
By using an inert gas like argon or nitrogen, the furnace creates an oxygen-free environment. This completely prevents oxidation, ensuring the material emerges from the heat treatment process with a clean, bright, and dimensionally accurate surface. This is critical for high-precision components.
Inducing Specific Chemical Changes
This is where atmosphere furnaces become a tool for material design, not just processing.
By introducing an active gas, you can deliberately alter the chemistry of the material's surface. A carbon-rich atmosphere can be used for carburizing, diffusing carbon into steel to create a very hard, wear-resistant outer case. This is a foundational process for creating gears and bearings.
Ensuring Structural and Mechanical Uniformity
Beyond chemistry, atmosphere furnaces provide an environment for exceptionally uniform heating.
This consistent temperature control, combined with a protective atmosphere, allows for processes like annealing. This refines the material's internal grain structure, relieves residual stresses from manufacturing, and results in more predictable and consistent mechanical properties like hardness and ductility.
Understanding the Trade-offs
While powerful, atmosphere furnaces introduce complexity that must be managed. Understanding these trade-offs is key to successful implementation.
Process Complexity and Cost
These systems are inherently more complex and expensive than standard air furnaces. They require gas storage, mixing panels, flow controllers, and safety systems for handling gases, which increases both capital investment and operational oversight.
Gas Purity and Flow Management
The effectiveness of the atmosphere is entirely dependent on its purity and stability. Leaks in the furnace can introduce oxygen, defeating the purpose of the inert gas. Likewise, incorrect gas flow rates can result in an incomplete purge or wasted gas, impacting both part quality and cost.
Process Development Time
Developing a robust and repeatable atmospheric process requires expertise. Dialing in the precise gas composition, flow rate, temperature, and time for a specific material and desired outcome is an engineering effort that requires careful testing and validation.
Making the Right Choice for Your Goal
The strategy you employ depends entirely on your end goal for the material.
- If your primary focus is surface integrity and purity: Use a high-purity inert gas atmosphere (like argon or nitrogen) to create a protective shield against oxidation.
- If your primary focus is enhancing bulk mechanical properties: Combine uniform heating with a protective atmosphere for annealing or stress-relieving to refine grain structure and improve consistency.
- If your primary focus is creating new surface properties: Utilize a specific reactive gas atmosphere to deliberately alter the material's surface chemistry, such as in carburizing or nitriding.
- If your primary focus is material synthesis and research: Use a precisely controlled atmosphere (which may include oxygen) to study material behavior or synthesize novel materials like battery cathodes.
Ultimately, mastering the furnace atmosphere is the key to unlocking a material's full potential.
Summary Table:
| Improvement Mechanism | Key Benefits | Common Applications |
|---|---|---|
| Preventing Surface Degradation | Eliminates oxidation and decarburization; maintains dimensional accuracy | High-precision components, clean surface finishes |
| Inducing Chemical Changes | Enhances surface hardness and wear resistance via carburizing or nitriding | Gears, bearings, tools |
| Ensuring Structural Uniformity | Refines grain structure; improves mechanical consistency and stress relief | Annealing, material synthesis, research |
Ready to enhance your material properties with precision? At KINTEK, we leverage exceptional R&D and in-house manufacturing to provide advanced high-temperature furnace solutions tailored for diverse laboratories. Our product line includes Muffle, Tube, Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems, all backed by strong deep customization capabilities to meet your unique experimental needs. Contact us today to discuss how our atmosphere furnaces can deliver superior strength, finish, and performance for your applications!
Visual Guide
Related Products
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
People Also Ask
- How does a chemically inert atmosphere function in a furnace? Prevent Oxidation and Ensure Material Purity
- How does nitrogen atmosphere heat treatment improve surface strengthening? Enhance Durability and Performance
- What is the use of nitrogen in furnace? Prevent Oxidation for Superior Heat Treatment
- What are the environmental benefits of using inert gases in furnaces? Reduce Waste and Emissions for a Greener Process
- What is nitrogen used for in a furnace? Prevent Oxidation and Control Heat Treatment Quality



















