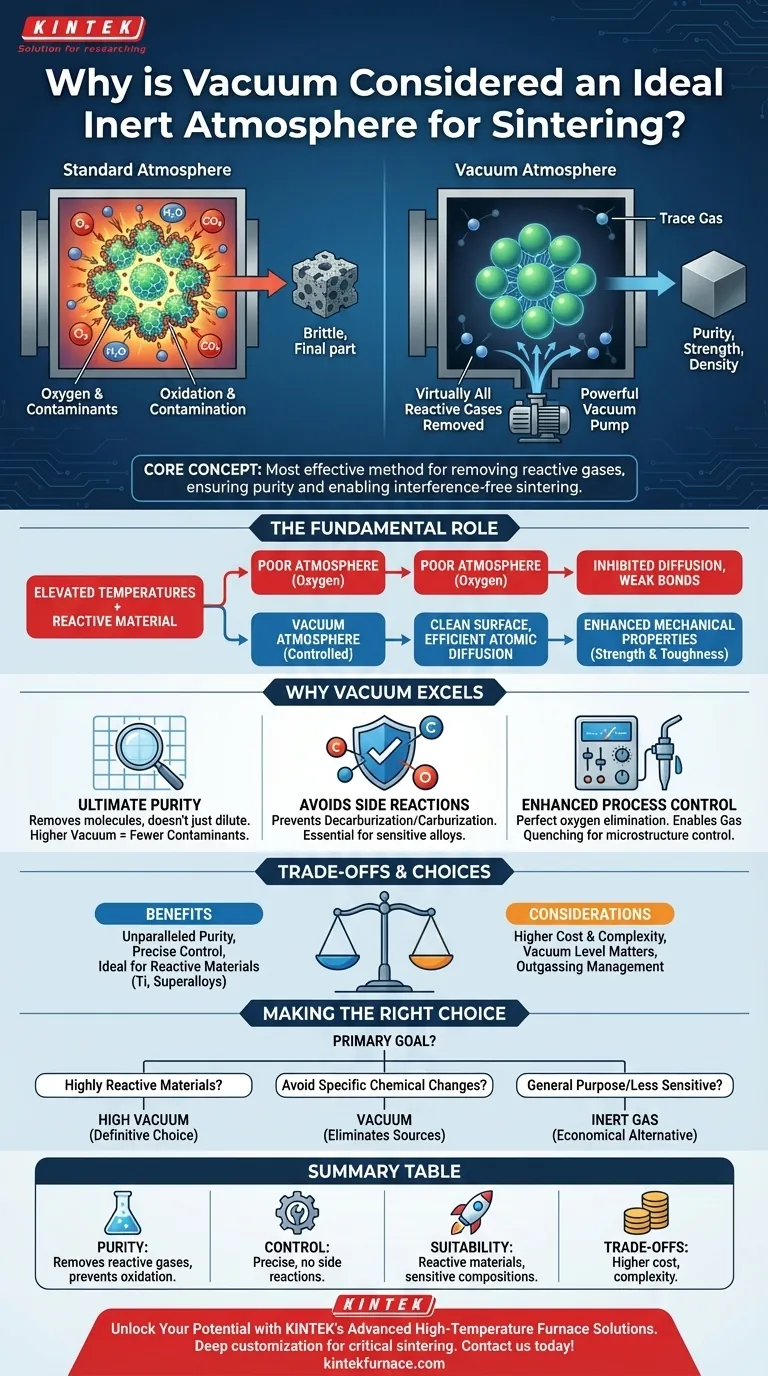
At its core, a vacuum is considered an ideal inert atmosphere for sintering because it is the most effective method for removing virtually all reactive gases from the furnace. By creating an environment devoid of oxygen and other potential contaminants, a vacuum prevents unwanted chemical reactions, ensuring the material's purity and allowing the fundamental sintering process to proceed without interference.
Sintering at high temperatures makes materials highly susceptible to oxidation and contamination. While inert gases can dilute reactive elements, a vacuum actively removes them, offering an unparalleled level of atmospheric control and purity that is critical for high-performance materials.

The Fundamental Role of Atmosphere in Sintering
Sintering involves bonding material particles together using heat, typically below the material's melting point. The atmosphere in which this occurs is not a passive element; it is an active variable that can either aid or hinder the entire process.
Preventing Oxidation and Contamination
At the elevated temperatures required for sintering, most materials become highly reactive. The primary threat is oxygen, which can form oxide layers on the particles, inhibiting the diffusion and bonding necessary for densification.
A controlled atmosphere is essential to prevent this. A vacuum or protective gas displaces the ambient air, protecting the material from oxidation and other forms of chemical contamination.
Enabling Sintering Reactions
Beyond just being protective, the right atmosphere can actively promote the desired sintering reactions. By removing gaseous byproducts and impurities from the material's surface, a clean environment allows for more efficient atomic diffusion between particles.
This results in a stronger, denser final part with enhanced mechanical properties like strength and toughness.
Why Vacuum Excels as an "Inert" Atmosphere
While inert gases like argon or nitrogen are commonly used, a vacuum provides distinct advantages, making it the superior choice for many critical applications.
Achieving Ultimate Purity
Even high-purity inert gases contain trace amounts of contaminants. A vacuum, by its nature, removes these molecules from the chamber rather than just diluting them.
The higher the degree of vacuum, the fewer molecules remain, creating an environment that is as close to a perfectly neutral atmosphere as possible. This minimizes any potential for reaction with the material being processed.
Avoiding Unwanted Side Reactions
A vacuum is uniquely suited for materials that are sensitive to specific gases. For example, some alloys are prone to decarburization (loss of carbon) or carburization (gain of carbon) in atmospheres containing carbon-based gases.
Because a vacuum contains no such elements, it entirely prevents these detrimental side reactions, a feat that is difficult to guarantee with other atmospheric controls. This makes it essential for processing materials with tightly controlled chemical compositions.
Enhancing Process Control
Modern vacuum furnaces provide perfect and repeatable control over the processing environment. They completely eliminate oxygen exposure, which is critical for oxidation-sensitive materials like titanium, refractory metals, and certain superalloys.
Furthermore, many vacuum furnaces integrate rapid cooling systems, such as gas quenching, allowing for precise control over the material's final microstructure after sintering is complete.
Understanding the Trade-offs
While powerful, a vacuum is not the universal solution for all sintering operations. Understanding its context is key to making a sound technical decision.
Vacuum Level Matters
Not all vacuums are created equal. The required vacuum level (low, medium, or high) depends entirely on the material's sensitivity to contamination. Achieving and maintaining a very high vacuum requires more sophisticated and costly equipment.
Cost and Complexity
Vacuum furnaces are generally more complex and expensive to procure and operate than furnaces that use a simple flowing inert gas. For less sensitive materials where basic oxidation prevention is the only goal, an argon atmosphere may be a more cost-effective solution.
Outgassing Considerations
During the initial pump-down phase, materials and furnace interiors can release trapped gases, a phenomenon known as outgassing. This must be properly managed to ensure the final vacuum level is sufficient for the process, sometimes requiring specific heating ramps or hold times.
Making the Right Choice for Your Goal
Selecting the correct atmosphere is a critical process decision that directly impacts the quality, performance, and cost of your sintered component.
- If your primary focus is processing highly reactive materials (e.g., titanium, superalloys): A high vacuum is the definitive choice to guarantee purity and prevent any unwanted oxidation or contamination.
- If your primary focus is avoiding specific chemical changes (e.g., decarburization): Vacuum is the ideal environment as it eliminates the source of the reacting elements that may be present in other atmospheres.
- If your primary focus is general-purpose sintering of less sensitive materials: A flowing inert gas like argon can provide adequate protection from oxidation and may be a more economical choice.
Ultimately, choosing a vacuum is a decision to prioritize material integrity and process control above all else.
Summary Table:
| Aspect | Vacuum Sintering Advantage |
|---|---|
| Purity | Removes virtually all reactive gases, preventing oxidation and contamination |
| Control | Enables precise process control with no unwanted side reactions like decarburization |
| Suitability | Ideal for highly reactive materials (e.g., titanium, superalloys) and sensitive compositions |
| Trade-offs | Higher cost and complexity compared to inert gas alternatives |
Unlock the full potential of your sintering processes with KINTEK's advanced high-temperature furnace solutions. Leveraging exceptional R&D and in-house manufacturing, we provide Muffle, Tube, Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems tailored to your unique experimental needs. Our strong deep customization capability ensures precise performance for critical applications like sintering reactive materials. Contact us today to discuss how we can enhance your lab's efficiency and material outcomes!
Visual Guide
Related Products
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- Molybdenum Vacuum Heat Treat Furnace
- Dental Porcelain Zirconia Sintering Ceramic Vacuum Press Furnace
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
People Also Ask
- What is the role of sintering or vacuum induction furnaces in battery regeneration? Optimize Cathode Recovery
- Why is a vacuum environment essential for sintering Titanium? Ensure High Purity and Eliminate Brittleness
- Why must sintering equipment maintain a high vacuum for high-entropy carbides? Ensure Phase Purity and Peak Density
- Why is a high-vacuum environment necessary in copper slag impoverishment? Maximize Your Matte Separation Efficiency
- What is the mechanism of a vacuum sintering furnace for AlCoCrFeNi2.1 + Y2O3? Optimize Your High-Entropy Alloy Processing



















