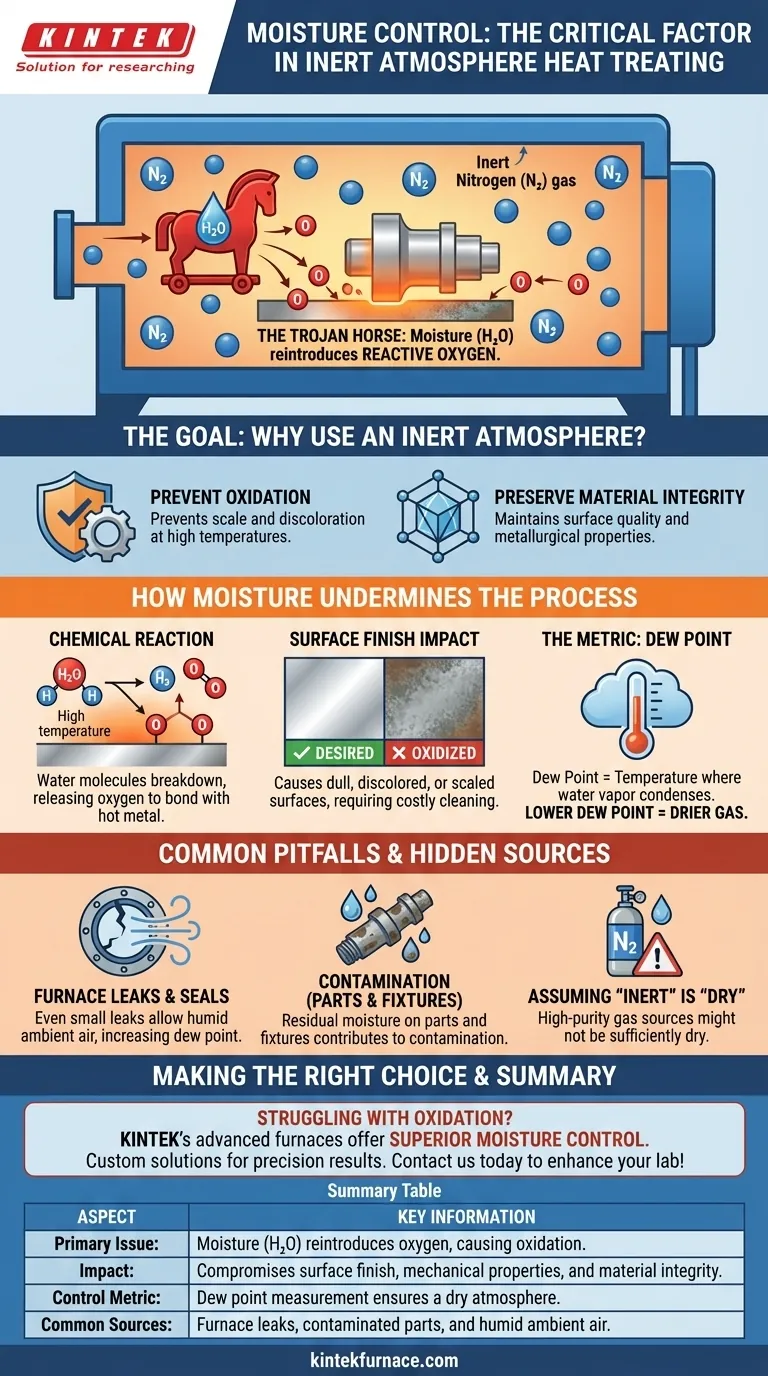
At its core, moisture control is critical in inert atmosphere heat treating because moisture (H₂O) acts as a Trojan horse, reintroducing reactive oxygen into a supposedly oxygen-free environment. Even with oxygen purged by an inert gas like nitrogen, the presence of water vapor at high temperatures will lead to oxidation, compromising the surface finish and mechanical properties of the material.
In an environment painstakingly cleared of oxygen, moisture becomes the primary source of oxidation. Controlling it by monitoring the dew point is not just a best practice; it is fundamental to the success of the entire process.

The Goal: Why Use an Inert Atmosphere?
Preventing Oxidation
The primary purpose of inert atmosphere heat treating is to prevent the metal from reacting with oxygen at elevated temperatures.
This reaction, known as oxidation, creates a layer of scale or discoloration on the part's surface. It can degrade the material's intended finish, dimensions, and performance characteristics.
Preserving Material Integrity
By replacing oxygen-rich air with a stable, non-reactive gas like nitrogen, the process preserves the material's surface quality and metallurgical structure, ensuring it meets precise engineering specifications.
How Moisture Undermines the Process
The Chemical Reaction at High Temperatures
While an inert gas like nitrogen displaces gaseous oxygen (O₂), it does not remove water vapor (H₂O).
At the high temperatures common in heat treating, water molecules become highly reactive. They can break down, releasing their oxygen atom to readily bond with the hot metal surface.
This is why the references state moisture "increases oxygen reactivity"—it provides a hidden, localized source of oxygen precisely where it can do the most damage.
The Impact on Surface Finish
The oxidation caused by moisture results in a dull, discolored, or scaled surface instead of the bright, clean finish typically desired.
This directly compromises the aesthetic and functional quality of the component, often requiring costly and damaging secondary cleaning operations like acid pickling or abrasive blasting.
The Metric for Control: Dew Point
The amount of moisture in an atmosphere is measured by its dew point.
Dew point is the temperature at which the water vapor in the gas would condense into liquid. A lower dew point signifies a drier gas with less moisture available to cause oxidation.
Monitoring and controlling the dew point of the furnace atmosphere is the most direct and reliable method for ensuring a sufficiently dry environment for successful heat treatment.
Common Pitfalls and Hidden Sources
Assuming "Inert" Gas is "Dry" Gas
A frequent mistake is to assume that using a high-purity inert gas is sufficient. While the gas source itself may be dry, moisture can be introduced from several other places.
Furnace Leaks and Seals
Even small leaks in furnace seals, doors, or fittings can allow humid ambient air to be drawn into the chamber, dramatically increasing the dew point and introducing both oxygen and moisture.
Contamination from Parts and Fixtures
Parts that are not thoroughly cleaned and dried before entering the furnace can carry residual moisture with them. The same applies to baskets, fixtures, and even the furnace's own refractory lining, which can absorb moisture from the air when cool.
Making the Right Choice for Your Goal
Achieving effective moisture control requires a holistic view of your entire process, not just the gas supply.
- If your primary focus is achieving a consistent, bright finish: Implement continuous, in-line dew point monitoring to establish a baseline and detect process deviations in real-time.
- If you are troubleshooting recurring oxidation issues: Investigate all potential moisture sources, including furnace integrity, part cleanliness, and ambient humidity, not just the inert gas specifications.
- If you are designing a new heat treatment cycle: Specify a maximum dew point requirement for your supplied inert gas and integrate dew point analysis as a critical quality control check from the outset.
Mastering moisture control transforms inert atmosphere heat treating from a variable art into a predictable, high-precision science.
Summary Table:
| Aspect | Key Information |
|---|---|
| Primary Issue | Moisture (H₂O) reintroduces oxygen, causing oxidation at high temperatures. |
| Impact | Compromises surface finish, mechanical properties, and material integrity. |
| Control Metric | Dew point measurement for monitoring and ensuring a dry atmosphere. |
| Common Sources | Furnace leaks, contaminated parts, and humid ambient air. |
Struggling with oxidation in your heat treatment processes? KINTEK's advanced high-temperature furnace solutions, including Muffle, Tube, Rotary, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems, are designed with superior moisture control features. Leveraging our exceptional R&D and in-house manufacturing, we offer deep customization to meet your unique experimental needs. Contact us today to enhance your lab's precision and efficiency!
Visual Guide
Related Products
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
People Also Ask
- What is the relationship between temperature and the furnace atmosphere in material processing? Master the Critical Heat-Environment Balance
- What industries commonly use inert atmosphere heat treating? Key Applications in Military, Automotive, and More
- How does the inert atmosphere heat treating process work? Prevent Oxidation for Superior Material Quality
- What are the benefits of inert atmosphere heat treating? Prevent Oxidation and Preserve Material Integrity
- What is the main purpose of heat treatment? Transform Metal Properties for Superior Performance



















