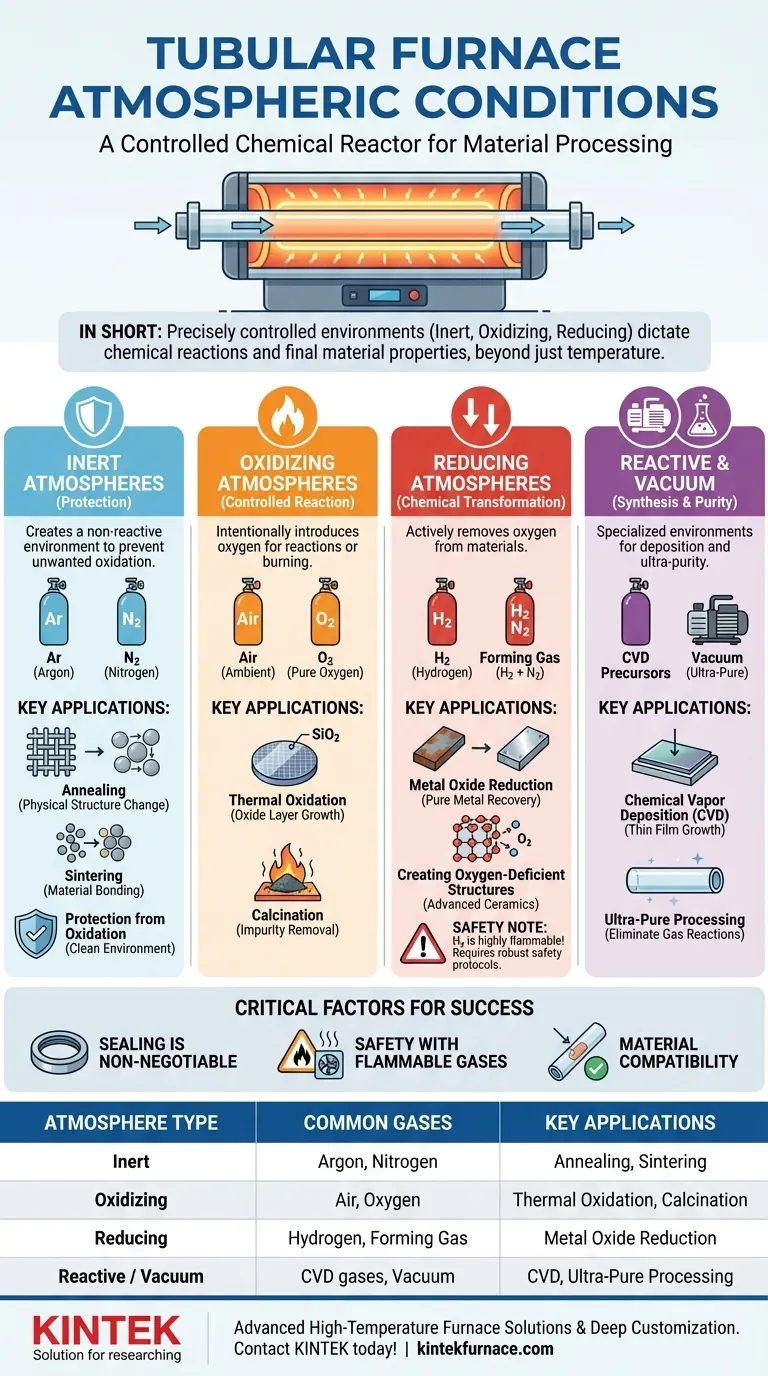
In short, a tubular furnace is designed to operate under a wide range of precisely controlled atmospheric conditions. The most common environments include inert atmospheres using gases like argon or nitrogen, oxidizing atmospheres using air or pure oxygen, and reducing atmospheres that typically involve hydrogen. This control is what makes the furnace a versatile tool for advanced material synthesis and processing.
The critical takeaway is that a tubular furnace is not merely a high-temperature oven; it is a controlled chemical reactor. The choice of atmosphere is an active process variable, just as important as temperature and time, which directly dictates the chemical reactions that occur and the final properties of your material.

The Role of Atmosphere in Material Processing
A common misconception is that a furnace's only job is to provide heat. In reality, at the high temperatures achieved in a tubular furnace, materials become highly reactive with any gases present.
Controlling the atmosphere allows you to either prevent unwanted reactions or deliberately drive a specific chemical transformation. Without this control, most processes would simply result in uncontrolled oxidation from the surrounding air.
A Breakdown of Common Furnace Atmospheres
The atmosphere you choose is entirely dependent on your desired outcome. Each type serves a distinct chemical purpose.
Inert Atmospheres (Protection)
An inert atmosphere is chemically non-reactive. Its purpose is to create a "clean" environment that protects the material from unwanted reactions, primarily with oxygen and water vapor from the air.
The most common inert gases are Argon (Ar) and Nitrogen (N2). Argon is heavier than air and completely inert, making it ideal for highly sensitive materials. Nitrogen is a cost-effective alternative for many applications where nitriding (formation of nitrides) is not a concern.
These are used for processes like annealing or sintering where the goal is to change a material's physical structure without altering its chemistry.
Oxidizing Atmospheres (Controlled Reaction)
An oxidizing atmosphere is used to intentionally introduce oxygen into a material's structure or to burn away organic compounds.
The simplest oxidizing atmosphere is ambient air. For processes requiring higher oxygen concentration or purity, pure Oxygen (O2) is used.
This is fundamental for applications like thermal oxidation, where a thin oxide layer is grown on a substrate (e.g., SiO₂ on a silicon wafer), or for calcination, where materials are heated to remove impurities or volatile substances.
Reducing Atmospheres (Chemical Transformation)
A reducing atmosphere is designed to do the opposite of an oxidizing one: it actively removes oxygen from a material.
These atmospheres almost always involve Hydrogen (H2), often used in a dilute, non-flammable mixture with nitrogen known as forming gas (e.g., 5% H₂ in 95% N₂).
This is critical for processes like reducing metal oxides back to their pure metallic form or for creating specific oxygen-deficient structures in advanced ceramics.
Reactive & Vacuum Atmospheres (Synthesis & Purity)
Beyond the main categories, tubular furnaces can handle more specialized environments.
Reactive atmospheres are used in processes like Chemical Vapor Deposition (CVD), where precursor gases react at high temperatures to deposit a solid thin film onto a substrate.
Vacuum is another crucial "atmosphere." By pumping the air out of the tube, you create an ultra-pure environment that eliminates nearly all potential gas-phase reactions. This is often the first step before back-filling the tube with a specific high-purity process gas.
Understanding the Trade-offs and Pitfalls
Achieving a truly controlled atmosphere requires more than just a gas cylinder. The integrity of your entire system is paramount.
Sealing is Non-Negotiable
Even a microscopic leak in your system will allow air to contaminate your process gas. When working with an inert or reducing atmosphere, this can ruin an experiment by introducing unwanted oxygen. Ensure all flanges, seals, and gas line connections are leak-tight.
Safety with Flammable Gases
Using hydrogen-based reducing atmospheres introduces a significant safety risk. Hydrogen is highly flammable. Any system using H₂ must have robust safety protocols, including proper ventilation, leak detection, and a system to safely burn off any unreacted exhaust gas.
Material Compatibility
The process tube itself (typically quartz, alumina, or mullite) and the sample material must be compatible with the process gases at the target temperature. For example, quartz tubes can be damaged by highly reducing conditions at very high temperatures.
Making the Right Choice for Your Goal
Select your furnace atmosphere based on the chemical transformation you intend to achieve.
- If your primary focus is to heat a material without chemical changes: Use an inert atmosphere like Argon or Nitrogen to prevent oxidation.
- If your primary focus is to grow an oxide layer or burn off contaminants: Use an oxidizing atmosphere like clean air or pure Oxygen.
- If your primary focus is to remove oxygen from a material: Use a reducing atmosphere like forming gas or pure Hydrogen, implementing all necessary safety measures.
- If your primary focus is to deposit a new thin-film material: Use a reactive gas mixture specifically designed for your CVD process in a leak-tight system.
Treating the furnace atmosphere as a deliberate reagent, not just a background condition, is the key to successful and repeatable high-temperature material processing.
Summary Table:
| Atmosphere Type | Common Gases | Key Applications |
|---|---|---|
| Inert | Argon, Nitrogen | Annealing, sintering, protection from oxidation |
| Oxidizing | Air, Oxygen | Thermal oxidation, calcination |
| Reducing | Hydrogen, Forming Gas | Reduction of metal oxides, creating oxygen-deficient structures |
| Reactive / Vacuum | CVD gases, Vacuum | Chemical vapor deposition, ultra-pure processing |
Leveraging exceptional R&D and in-house manufacturing, KINTEK provides diverse laboratories with advanced high-temperature furnace solutions. Our product line, including Muffle, Tube, Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems, is complemented by our strong deep customization capability to precisely meet unique experimental requirements. Achieve superior material synthesis with precise atmospheric control—contact us today to discuss how our furnaces can enhance your research and processing outcomes!
Visual Guide
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- Multi Zone Laboratory Quartz Tube Furnace Tubular Furnace
- Split Multi Heating Zone Rotary Tube Furnace Rotating Tube Furnace
People Also Ask
- What recent improvements have been made to lab tube furnaces? Unlock Precision, Automation & Safety
- Why is a tube furnace utilized for the heat treatment of S/C composite cathode materials? Optimize Battery Stability
- How is a high-temperature tube furnace utilized in the synthesis of MoO2/MWCNTs nanocomposites? Precision Guide
- What are the key operational considerations when using a lab tube furnace? Master Temperature, Atmosphere & Safety
- What role does a laboratory tube furnace perform during the carbonization of LCNSs? Achieve 83.8% Efficiency



















