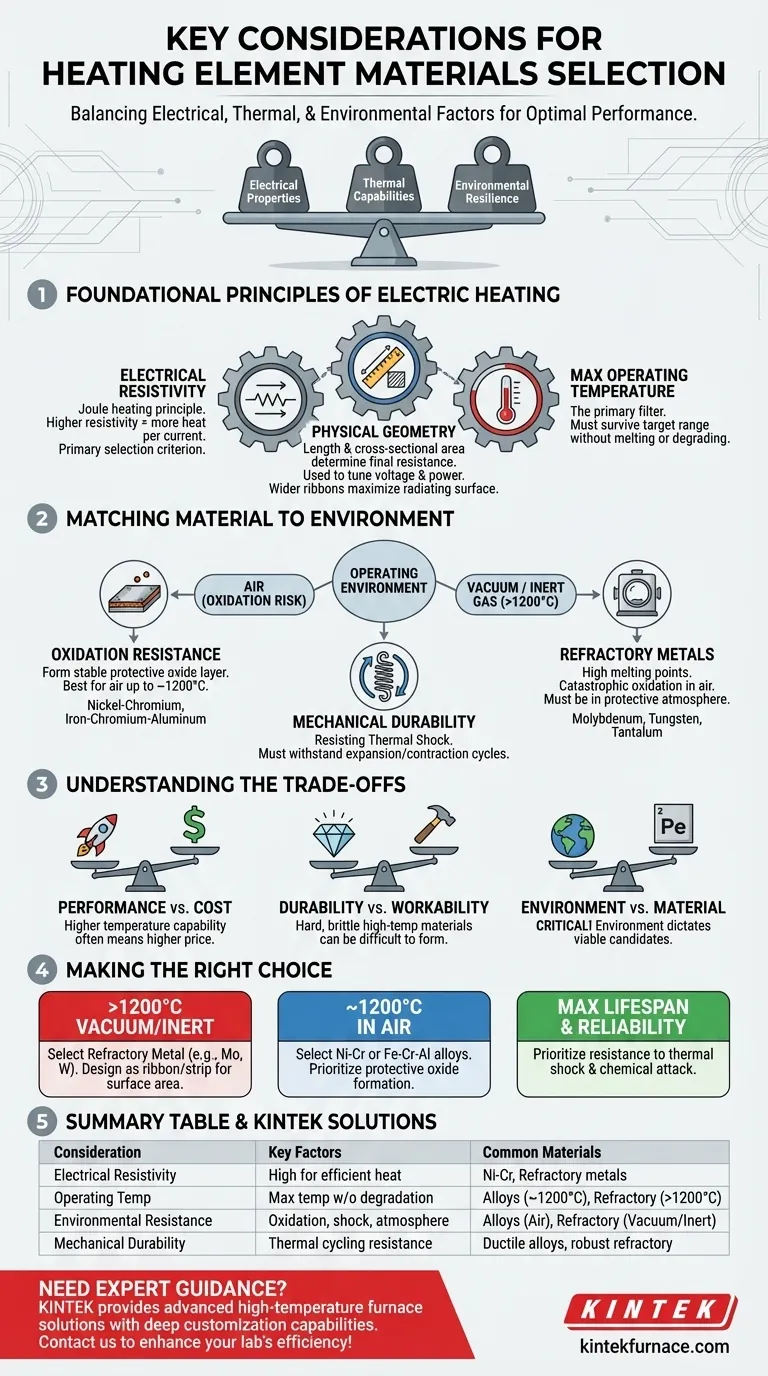
Selecting the right material for a heating element is a critical engineering decision that balances electrical properties, thermal capabilities, and environmental resilience. The ideal choice must have sufficient electrical resistance to generate heat effectively, withstand the target operating temperature without degrading, and survive the chemical and mechanical stresses of its environment, such as oxidation and thermal shock.
The optimal heating element is not just a material, but a complete system. Success depends on harmonizing the material's intrinsic properties (like resistivity) with its physical geometry and the specific operating environment it will face.

The Foundational Principles of Electric Heating
Understanding the core physics is the first step toward making an informed choice. The material you select is governed by these fundamental constraints.
Electrical Resistivity: The Engine of Heat
All electric heating is based on the principle of Joule heating, where electrical energy is converted into thermal energy as current passes through a resistor. A material with higher electrical resistivity will generate more heat for a given current and physical dimension.
This makes resistivity a primary selection criterion. Materials are chosen specifically for their ability to impede electrical flow and convert that energy into useful heat.
Physical Geometry: Tuning the Performance
A material's intrinsic resistivity is only part of the equation. The element's final resistance is determined by its physical shape—specifically its length and cross-sectional area.
A wider, thicker ribbon or a larger diameter wire has a lower overall resistance than a thinner one of the same material. Designers use this principle to "tune" an element for a specific voltage and power output. For example, in vacuum furnaces, wide ribbons are often used not only to adjust resistance but also to maximize the radiating surface area, improving heat transfer efficiency.
Maximum Operating Temperature: The Primary Filter
The single most important factor is the required operating temperature. Every material has a maximum temperature at which it can operate reliably before it melts, rapidly oxidizes, or loses its structural integrity.
This factor acts as the first and most rigid filter. You must begin by shortlisting only those materials capable of surviving your target temperature range.
Matching the Material to the Environment
A material that performs perfectly in one environment can fail catastrophically in another. The application and operating atmosphere are just as important as the temperature.
Oxidation Resistance: The Fight Against Air
When heated in the presence of oxygen, most metals will react and form an oxide layer. For a heating element, this can be destructive, causing it to thin, increase in resistance, and eventually burn out.
Some materials, like nickel-chromium alloys, are designed to form a stable, adherent oxide layer that protects the underlying metal from further attack. This makes them ideal for applications in open air.
High-Vacuum Performance: Refractory Metals
In high-temperature applications above 1200°C, especially in vacuum or inert gas atmospheres, refractory metals are the standard. Materials like molybdenum, tungsten, and tantalum offer extremely high melting points.
However, these metals oxidize catastrophically at high temperatures in air and therefore must be used in a vacuum or a protective, oxygen-free atmosphere.
Mechanical Durability: Resisting Thermal Shock
Heating elements are subjected to constant expansion and contraction as they cycle on and off. This thermal shock can cause materials to become brittle, crack, or deform over time.
A good heating element material must possess sufficient ductility and mechanical strength to withstand thousands of these cycles without failing, ensuring a long and reliable service life.
Understanding the Trade-offs
There is no single "best" material, only the most appropriate one for a given set of constraints. Every choice involves balancing competing factors.
Performance vs. Cost
Higher performance almost always comes at a higher price. Refractory metals like tungsten and tantalum, which can operate at extreme temperatures, are significantly more expensive than common alloys like nickel-chromium. You must justify the need for higher temperature capability against the project's budget.
Durability vs. Workability
Some of the most durable high-temperature materials can be very hard and brittle at room temperature. This can make them difficult to form into complex shapes and require more careful design for mounting and support to prevent mechanical failure.
Environment vs. Material
This is the most critical trade-off. You cannot use an otherwise ideal high-temperature material like molybdenum in an open-air furnace because it will simply burn away. The operating environment dictates the list of viable candidates before any other factor is considered.
Making the Right Choice for Your Application
Use your primary goal to guide your material selection process.
- If your primary focus is high-temperature heating (>1200°C) in a vacuum or inert gas: Select a refractory metal like molybdenum or tungsten, and design the element as a ribbon or strip to maximize radiant surface area.
- If your primary focus is general-purpose heating in air (up to ~1200°C): Your best choice will be a nickel-chromium or iron-chromium-aluminum alloy designed to form a protective oxide layer.
- If your primary focus is maximizing element lifespan and reliability: Look beyond maximum temperature and prioritize materials with proven resistance to thermal shock and chemical attack within your specific operating environment.
By understanding these core principles, you can design a robust heating system that is efficient, reliable, and perfectly suited to its task.
Summary Table:
| Consideration | Key Factors | Common Materials |
|---|---|---|
| Electrical Resistivity | High resistivity for efficient heat generation | Nickel-chromium alloys, refractory metals |
| Operating Temperature | Maximum temperature without degradation | Up to ~1200°C for alloys, >1200°C for refractory metals |
| Environmental Resistance | Oxidation, thermal shock, and atmosphere compatibility | Alloys for air, refractory metals for vacuum/inert gas |
| Mechanical Durability | Resistance to thermal cycling and physical stress | Ductile alloys, robust refractory metals |
Need expert guidance on selecting the perfect heating element for your lab? At KINTEK, we leverage exceptional R&D and in-house manufacturing to provide advanced high-temperature furnace solutions. Our product line includes Muffle, Tube, Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems, all backed by strong deep customization capabilities to precisely meet your unique experimental requirements. Contact us today to enhance your lab's efficiency and reliability with tailored heating solutions!
Visual Guide
Related Products
- Molybdenum Vacuum Heat Treat Furnace
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
People Also Ask
- What is the purpose of setting a mid-temperature dwell stage? Eliminate Defects in Vacuum Sintering
- What tasks does a high-temperature vacuum sintering furnace perform for PEM magnets? Achieve Peak Density
- Why is a vacuum environment essential for sintering Titanium? Ensure High Purity and Eliminate Brittleness
- What role does a high-temperature vacuum heat treatment furnace play in TBC post-processing? Enhance Coating Adhesion
- What are the benefits of using a high-temperature vacuum furnace for the annealing of ZnSeO3 nanocrystals?



















